Scoring prognostic factors for pregnancy post-intrauterine insemination in couples with infertility
Geraldo Laurus, Agustinus Agustinus, Cennikon Pakpahan, Vellyana Lie, Maitra Djiang Wen
Corresponding author: Cennikon Pakpahan, Department of Biomedical Science, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia 
Received: 17 Nov 2024 - Accepted: 17 Feb 2025 - Published: 03 Mar 2025
Domain: Obstetrics and gynecology
Keywords: Intrauterine insemination, prognostic factors, pregnancy, scoring, reproductive health
©Geraldo Laurus et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Geraldo Laurus et al. Scoring prognostic factors for pregnancy post-intrauterine insemination in couples with infertility. Pan African Medical Journal. 2025;50:64. [doi: 10.11604/pamj.2025.50.64.45962]
Available online at: https://www.panafrican-med-journal.com//content/article/50/64/full
Research 
Scoring prognostic factors for pregnancy post-intrauterine insemination in couples with infertility
Scoring prognostic factors for pregnancy post-intrauterine insemination in couples with infertility
Geraldo Laurus1, Agustinus Agustinus2,3, ![]() Cennikon Pakpahan2,3,&, Vellyana Lie2,4, Maitra Djiang Wen1,4
Cennikon Pakpahan2,3,&, Vellyana Lie2,4, Maitra Djiang Wen1,4
&Corresponding author
Introduction: intrauterine insemination (IUI) was selected as a treatment for infertility due to its simple method, affordability, and non-invasive. Intrauterine insemination had pregnancy rates after one cycle of IUI ranging between 8.2% and 15.1%. Candidates for IUI are couples facing specific infertility including cervical factors, ovulatory dysfunction, endometriosis, and ejaculation. Therefore, this study aimed to assess prognostic factors significantly affecting the outcome of IUI in Indonesia before the IUI procedure.
Methods: this study was conducted at Bocah Indonesia Fertility Clinic, Tangerang, and SMART IVF Anna Hospital, Bekasi, using an observational analytic method with a cohort retrospective design. Data were collected from infertile couples subjected to IUI between 2020 and 2024. The primary outcome measured was biochemical pregnancy, defined as a positive serum human chorionic gonadotropin (hCG) test following IUI. Prognostic factors for pregnancy were assessed and identified, while a predictive scoring system was developed using a multivariate analysis.
Results: a total of 443 IUI cycles were evaluated and a pregnancy rate of 13.5% was observed. Multivariate analysis showed that pregnancy rates were influenced by the body mass index (BMI) of the male (<27.5 kg/m2), age of the female (<30.5 years old), total progressive sperm count after sperm preparation (>15.81 million), first insemination, anti-mullerian hormone (AMH) level (>3.015 ng/mL), and endometrial thickness (>9.36 mm). A scoring system ranging from 0-9 was developed, with a probability of pregnancy from 1.31% to 55.16%, for scores 0 to 9, respectively.
Conclusion: the scoring system served as a tool designed specifically to evaluate the relationship between prognostic factors and post-IUI pregnancy rates.
Intrauterine Insemination (IUI) was selected as a treatment for infertility due to the simple method, affordability, and non-invasive nature compared to other procedures namely in vitro fertilization (IVF) [1-3]. Furthermore, this procedure is relatively safe as there are no associated significant complications [4]. Intrauterine insemination (IUI) can cause various factors such as cervical factors, ovulatory dysfunction, endometriosis, immunological causes in women, ejaculation and coital disorders in men, as well as unexplained infertility.
Several studies have shown that pregnancy rates after one cycle of IUI ranged between 8.2% and 15.1% [5-10]. There were several prognostic factors contributing positively to pregnancy rates. Prognostic factors can be divided into pre-cycle, insemination, and post-cycle factors. Pre-cycle prognostic factors in men, such as age [11] total motility, and normal morphology using strict criteria [7]. Pre-cycle prognostic factors from the female aspect were female age [4,5,7,12,13], endometrial thickness [5,14], the number of mature follicles after stimulation [5,7,15], duration of infertility [7,14,16], and the stimulation protocol used for IUI [5,7,12]. Factors during insemination such as obstruction during catheter placement, abdominal cramps, catheter bleeding, and bleeding during insemination can affect pregnancy rates [17]. Measures facilitating catheter placement were observed to have a positive influence [18]. Administration of progesterone preparations as luteal support is a post-cycle factor that can also affect pregnancy rates [19].
Several studies have evaluated prognostic factors for this treatment success. However, none have analyzed the factors for post-IUI pregnancy rates in the Indonesian population. The results of some investigations conducted in the absence of couples with infertility showed a scoring model for prognostic factors of post-IUI pregnancy rates [20,21]. This study aims to evaluate the relationship between prognostic factors and post-IUI pregnancy rates. The result is expected to be a scoring model to assess rates that are specific and individualistic.
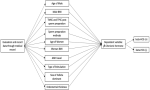
Study design: an observational analysis with a retrospective cohort design was adopted to evaluate the relationship between prognostic factors for pregnancy rates in post-IUI patients (Figure 1).
Setting: the population of this study was medical records of patients with infertility who sought treatment at Bocah Indonesia Fertility Clinic and SMART IVF Anna Hospital, as well as experienced IUI during the period from 2019 to December 2023.
Participants: the participants in this study are medical records of the patients who took IUI Bocah Indonesia Fertility Clinic and SMART IVF Anna Hospital. The criteria comprised data related to IUI in the medical records of couples with infertility who experienced IUI procedures at Bocah Indonesia Fertility Clinic and SMART IVF Anna Hospital Bekasi. We excluded the IUI data containing prognostic factors in incomplete medical records.
Variables: there are ten independent variables in this study and one dependent variable. Ten independent variables are the age of the male or female, BMI male or female, TMSC (total motility sperm count) and TPSC (total progressive sperm count) post sperm preparation, sperm preparation method, AMH (anti-mullerian hormone level), type of stimulation, size of the follicle, and endometrial thickness. TMSC refers to multiplying the percentage of motile spermatozoa (rapidly progressive, slowly progressive, and non-progressive) by the volume and concentration before sperm preparation. Total progressive sperm count (TPSC) is the percentage of progressively motile spermatozoa multiplied by the volume and concentration obtained after sperm preparation. Meanwhile, the dependent variable is the beta-chorionic hormone level, with 10 IU as the threshold for pregnancy. This pregnancy was defined as a biochemical pregnancy. We just assessed biochemical pregnancy in this study.
Data sources and measurement
Data collection: the data we extracted from the corresponding medical records included the couple's age, anthropometry, sperm profile, hormone levels, type of female stimulation used, follicle count from ultrasound, and endometrial thickness. All these data were considered as independent variables. We then extracted the patient's pregnancy data by pulling the beta-HCG level data as the dependent variable. All data extraction sheets were designed and used to collect data.
Sample size: the sample size is based on the rule of thumb with the formula:
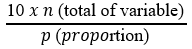
Eleven variables will be evaluated, and the incidence of subjects with the result of acceptable pregnancy was 30% [22]:
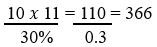
Sampling strategy: the method adopted was total sampling, comprising all samples that met the study criteria and fulfilled the inclusion and exclusion criteria.
Data analysis: this study's data analysis process consisted of six steps using Statistical Package for the Social Sciences (SPSS) version 25.0 (Figure 2). We first conducted a univariate analysis to get the descriptive values or profiles of the participants. Then, conduct a bivariate analysis between independent and dependent variables. Each independent variable that was previously a numeric scale will be determined first with area under the curve (AUC), receiver operating characteristic (ROC), and Youden test analysis. Then, the analysis continued with the logistic regression test by including each bivariate analysis test result with a value of p < 0.25 to calculate the score value of each variable.
Ethical considerations: this study had ethical approval and was approved by the University of Airlangga Ethics Committee (339/EC/KEPK/FKUA/2023).
Participant descriptive
Demography and clinical characteristics: samples comprised medical records of infertile couples who had experienced the IUI procedure at Bocah Indonesia Fertility Clinic, Tangerang, and SMART IVF Anna Pekayon Hospital, Bekasi. The size was 443 couples, of which 60 were fertile (13.5%). The participants’ characteristics are presented in Table 1 which shows several differences in characteristics between pregnant and non-pregnant after the administration of IUI. The mean ages of male partners were 31 and 32.9 years, respectively, indicating a significant difference. In the female partners, the mean ages of the 2 groups were 29.6 and 30.9 years. However, the male and female BMI did not show a significant difference. In terms of TMSC and TPSC post-preparation, couples who successfully conceived with p values 0.021. Furthermore, female factors such as AMH levels and endometrial thickness showed p values < 0,001, and higher pregnancy success. Other factors such as the number of follicles and the size of mature follicles presented no significant differences (p=0.536 and p=0.955). Additionally, differences in sperm preparation methods, number of inseminations, etiology of female infertility, and type of stimulation were not significantly different between the groups (p>0.05).
Outcome data
Receiver operating characteristic analysis determining cutoff variables: receiver operating characteristic analysis was conducted to determine the scoring of prognostic factors for pregnancy in infertile couples after undergoing IUI. Statistical analysis aimed to evaluate the cutoff values for each variable generated from the AUC curve. Figure 2 showed the results of ROC analysis, AUC, as well as the sensitivity and specificity values of the cutoff for several numeric variables such as the age of male and female, BMI of male and female, TMSC and TPSC post-preparation, AMH levels, number of follicles, size of mature follicles, and endometrial thickness. The results of ROC analysis of all prognostic factors evaluated showed that AMH levels (AUC = 0.667; p<0.001) and endometrial thickness (AUC = 0.651; p<0.001) had the highest AUC values. The sensitivity and specificity values were statistically found slighter than 70% using the regression logistic analysis test. Other factors such as male age, female age, and post-preparation TMSC and TPSC also produced statistically significant ROC (p<0.05).
After analysis ROC and AUC continue to Table 2 shows the bivariate analysis between prognostic factors divided based on cutoff values and pregnancy post-IUI. A significant relationship was observed between AMH levels and endometrial thickness (p<0.001) with pregnancy. Total motility sperm count values were also associated with post-IUI pregnancy in infertile couples (p=0.027). Other variables that show a significant relationship in bivariate analysis were male age, female age, and BMI of males with post-IUI pregnancy (p=0.041, p=0.042 and p=0.036).
Multivariate analysis of male and female prognostic factors in pregnancy: multivariate analysis was conducted on all prognostic factor variables with p<0.25 in the bivariate analysis. The analysis adopted the multiple logistic regression analysis procedures and the backward stepwise multivariate binary logistic regression method. The categorical variables included were the age of male and female, BMI of male and female, TPSC post-preparation, AMH levels, endometrial thickness, insemination number, as well as stimulation type. Variables with p<0.1 in the multivariate analysis were considered significant prognostic factors related to post-IUI pregnancy in the scoring system. Table 3 shows the results of the multivariate analysis of prognostic factors for post-IUI pregnancy. The results of the multivariate analysis using the backward stepwise multivariate binary logistic regression method showed that the prognostic scoring model for post-IUI pregnancy included variables such as male BMI, female age, TPSC, AMH levels, endometrial thickness, and first insemination. These variables were identified as significant prognostic factors (p<0.10).
Determining the scoring value of each variable: scoring values for each variable were determined based on the regression coefficient (β) and standard error obtained from the multiple logistic regression model. The updated scoring values for both male and female prognostic factors are presented in Table 4. The total prognostic factor score, based on scoring results, ranged from 0 to 9. In this study, infertile couples who became pregnant after IUI had an average total score between 5 and 6, while those who did not achieve pregnancy had an average score of around 4. Table 5 presents the results of the comparative test of total scores between pregnant and non-pregnant groups. A significant difference (p<0.001) was observed based on the comparative test of the total prognostic factor scores for post-IUI pregnancy.
This study aims to evaluate several prognostic factors from both males and females concerning pregnancy outcomes post-IUI among infertile couples in Indonesia. Couples subjected to IUI with complete medical records were included as samples. Prognostic factors used were drawn from previous studies [5,12,14,15,20]. The factors are routinely evaluated before IUI procedures. Those significantly associated with post-IUI pregnancy and included in the scoring system of this study were divided into pre-cycle (female age, male BMI, AMH levels), insemination (TMSC and endometrial thickness), and post-insemination (TPSC post preparation and number of insemination). Others comprising factors pre-cycle, such as male age and female BMI, etiology of infertility in women; and insemination, such as sperm preparation method, type of ovarian stimulation, and the size and number of mature follicles, were not significantly associated with post-IUI pregnancy and were omitted from the scoring system.
The success rate of IUI in this study was approximately 13.5%, comparable with reported values between 8.2% and 15.1% (7-9). In the demographic analysis and frequency distribution of a sample, the average age of males and females subjected to IUI was 32.7 and 30.8 years, respectively. The age is slightly younger compared to some previous investigations [2,13,14,23]. This could be attributed to the younger average age of marriage in Indonesia [24]. Supporting the observation, Bennett et al. stated that the average age of females attending fertility clinics was 26 years [25].
The average BMI of females in this study was 24 kg/m2, similar to the general value in Indonesia, according to a 2023 study [26]. However, it is lower compared to other studies of females subjected to IUI. Studies by Soria et al. and Muthigi et al. reported an average BMI of 26 kg/m2 [4,12]. This difference could be due to the generally higher average BMI in European and American populations, where the investigations were conducted [27]. Supporting the observations, a 2023 meta-analysis and systematic review showed that female BMI was not significantly associated with pregnancy outcomes post-IUI [28]. Meanwhile, data on the relationship between male BMI and post-IUI pregnancy rates remains limited. Existing studies stated that obesity in males was associated with decreased sperm quality, but only a few examined the impact on post-IUI outcomes [29]. The relationship between male BMI and pregnancy is often indirect. In obese males, increased leptin levels due to higher lipogenesis in adipose cells disrupt the hypothalamic-pituitary-testicular axis, impairing testosterone production, which is crucial for spermatogenesis [30]. Additionally, oxidative stress, such as elevated levels of O2-, is increased in obese males, negatively affecting sperm quality [31].
Other prognostic factors that were significantly associated with post-IUI pregnancy were AMH and endometrial thickness. The mean AMH value was higher compared to a previous study by Luo et al. [20] Anti-Müllerian hormone (AMH) is secreted by granulosa cells within the ovarian follicles, and its levels naturally decrease with maternal age. A reduced ovarian reserve is associated with a significantly lower likelihood of achieving a clinical pregnancy (CP). While AMH is recognized as a marker of ovarian reserve in individuals undergoing fertility treatment, its levels can serve as a guide for treatment expectations and management. Numerous studies on AMH have demonstrated a correlation between AMH levels and ovarian response to stimulation [32].
Factor insemination, like endometrial thickness, has been proven to be significantly correlated to post-IUI pregnancy in other studies (5,14). Factors that had no association were sperm preparation method, type of ovarian stimulation or induction, and the size and number of mature follicles. This is consistent with the report where the relationship between the factors and post-IUI pregnancy rates varies significantly [12,13,33]. Male factor most studied about post-IUI pregnancy is the total number of motile sperm inseminated. Existing reports show that motile sperm count is significantly associated with post-IUI pregnancy [5,7,8,21]. However, the minimum threshold post-preparation capable of enhancing the probability of pregnancy post-IUI remains uncertain. Michau et al. stated that a TMSC of more than 5 million is associated with higher pregnancy rates [8], while in the study conducted by Gubert et al. the same threshold of 5 million was significant only for males under 35 years old [7]. Another study by Muthigi et al. recommended a higher minimum threshold of 9 million [12]. The suggested cutoff value post-preparation was 15.81 million. The lowest TPSC in couples who became pregnant post-IUI was 5.01 million.
The results showed that the first insemination is a significant prognostic factor for IUI success. However, second or subsequent inseminations in the same couple had lower success rates compared to the first. This differs from previous investigations, where the second and third inseminations had higher pregnancy rates compared to the first. Muthigi et al. and Soria et al. stated that increasing the number of IUI cycles up to the second or third significantly increased fertility [4,12]. Another study conducted by Zippl et al. evaluated the success rates of the administered treatment. The results showed a higher increase where the probability of pregnancy could more than triple by the sixth insemination compared to the first [21]. However, physiological factors can influence the decrease in success rates of second and subsequent cycles. IUI as an infertility therapy has considerable costs and high uncertainty, leading to increased stress for both partners. Studies have shown that stress levels increase significantly after unsuccessful infertility treatment [34]. The failure of the administered therapy can also increase the incidence of depression, which, alongside psychological stress, affects the success of subsequent cycles [23]. Differences in results in this study can be attributed to a small number of couples experiencing 3 to 4 IUI cycles. The higher pregnancy rates in the first treatment should be considered before deciding on subsequent cycles in cases of failure. It is important to recognize that pregnancy rates after the second or subsequent IUI may not increase the probability of success compared to the first. The probability may increase when other modifiable prognostic factors are significantly improved before the next cycle. The second IUI increases costs and psychological burden; hence, it should only be recommended when proven to be more effective [35].
The etiology of female infertility in this study was not related to post-IUI pregnancy rates. Furthermore, it was divided into 4, namely none or unknown, PCOS, endometriosis, and uterine disorders. Other studies analyzing the relationship between the etiology of infertility and pregnancy rates post-IUI also showed mixed results. Luo et al. Gubert et al. and Huniadi et al. stated that the etiology of infertility was not significantly related to post-IUI pregnancy rates [7,14,20]. Meanwhile, Michau et al. and Dinelli et al. [5,8] identified a significant relationship. The difference in results could be attributed to the use of very diverse etiological classifications of infertility. Gubert et al. categorized Infertility into unexplained problems in males, females, or both without specifically subdividing female infertility etiologies [7]. Huniadi et al. classified infertility into male, female, a combination of both, and unexplained [14]. Michau et al. divided the etiological factors of female into ovulation, cervical disorders, endometriosis, and low ovarian reserve [8]. Meanwhile, Dinelli et al. shared the same factors as Michau et al. without low ovarian reserve classification [5,8]. The etiology of female infertility, which was not significantly related to post-IUI pregnancies, was attributed to differences in selecting the partner to be subjected to insemination-based indications. Since each fertility clinic has highly varied protocols and clinical pathways, there is a possibility that moderate or severe etiologies of female infertility will not be inseminated. The background of female infertility conditions, which may be less diverse, causes the etiology significantly related to post-IUI pregnancy [36].
Other factors influencing clinical pregnancy outcomes are insulin resistance, LH, FSH, testosterone, and estradiol. In women, insulin resistance may affect oocyte quality by damaging oocytes through telomeres and spindle disruption, leading to embryonic development arrest, impaired endometrial tolerance, recurrent miscarriage, and inflammatory responses [37]. In men, insulin resistance can impact the concentration and production of testosterone, reduce semen volume, and decrease the percentage of progressively motile sperm [38]. The LH, FSH, testosterone, and estradiol levels in men can influence spermatozoa quality. Deoxyribonucleic acid (DNA) fragmentation analysis should be considered as a predictor of insemination outcomes, as sperm deoxyribonucleic acid (DNA) damage can lead to a reduced success rate of pregnancy [39]. However, these factors may be considered less practical as the necessary testing facilities are not always available in all healthcare centers, making it difficult to consider them in scoring factors in IUI.
Comparison with other post-IUI pregnancy prognostic factor scoring system studies: the outcome of this study is a prognostic factor scoring system consisting of male BMI, female age, TPSC post-preparation, AMH levels, endometrial thickness, and first insemination. Each prognostic factor is assigned a score ranging from 1 to 3, with a total score ranging from 0 to 9. Each total score corresponds to a different probability of post-IUI pregnancy. References were drawn from 2 investigations with prognostic factor scoring system outcomes in 2021 and 2022. The study by Luo et al. (2021) focused on prognostic factors for post-IUI pregnancy among infertile couples in China [20]. Due to comparable success rates (13.6% in Luo et al. study and 13.0% in our study) and equivalent statistical methods, specifically backward stepwise multivariate binary logistic regression, prognostic factors included in the final scoring system were stimulation type (natural cycle or stimulated cycle), female age, single or double IUI, as well as parameters related to sperm motility and morphology obtained through computer-assisted sperm analysis (CASA) [20]. Meanwhile, more comprehensive and specific parameters for sperm motility and morphology evaluation were provided. The prognostic factor scoring of this study was more comprehensive because it included additional factors such as male BMI, AMH levels, and endometrial thickness. The evaluated TPSC in the investigation conducted by Luo et al. [20]. was unrelated to post-IUI pregnancy. On the contrary [7,8,12,14,33], stated that total post-preparation motile sperm count was a significant prognostic factor for pregnancy. Another comparable aspect was the use of a user-friendly and less practical scoring system. The outcome was in the form of formulas where each numerical value needed to be inputted individually and calculated manually, facilitating errors and less straightforwardness.
The latest report on prognostic factors for post-IUI pregnancy, using a comparative scoring system, is the study by Zippl et al. [21]. This study recorded an overall pregnancy rate of only 10.9%. Furthermore, the clinical scoring system includes factors such as female age, TPSC, female infertility etiology, and AMH levels. Other factors such as female BMI were also evaluated in this study but not included in the final scoring. However, BMI, type of stimulation, mature follicles, and endometrial thickness were not evaluated in this study. The scoring system developed is user-friendly, allowing inputs of 0, 1, or 2 for each prognostic factor. The inputs were then summed and adjusted to determine the chances of post-IUI pregnancy directly. This ease of use has led to the adoption of a similar scoring system, enhancing the applicability of the assessment. The highest success probability reported in the Australian study exceeded 60%, which is above the 55.16% reported in this study. A significant difference between the studies lies in the determination of cutoff values. For AMH, TPSC, and BMI parameters, the values used are not based on the characteristics of the sample population and are derived from studies not comprising Indonesians, hence becoming less suitable for adoption. In this study, the cutoff values are determined based on statistical tests, allowing for a better depiction of the characteristics of infertile couples.
The difference between this study and the 2 prognostic factor scoring investigations conducted by Luo et al. and Zippl et al. [20,21] lies in the parameter of TPSC used. This study evaluates post-preparation sperm parameters, while the previous 2 applied TPSC and TMSC values before preparation. Post-preparation TPSC was evaluated in prognostic factor scoring because this sperm was inseminated indirectly, hence, more specifically representing the sperm factor in males. The 2 studies also used populations in Austria and China. Furthermore, the differences in backgrounds and characteristics compared to the Indonesian population are factors that should be considered when adopting a scoring system. This presents the importance of developing prognostic factor scoring for post-IUI pregnancy with populations of infertile couples in Indonesia, as well as cutoff values and parameters that can specifically represent the population. The user-friendly and applicable output of the scoring system enables rapid and holistic use for educational purposes and decision-making. Additionally, prognostic factors from both the male and female sides should be carefully and equally considered before making decisions for IUI.
Limitations of this study: this study was observational with a retrospective cohort design, utilizing data from medical records. Based on this design, certain prognostic factors related to post-IUI pregnancy, which are not recorded in medical records, cannot be evaluated. Examples of these factors include difficulties encountered during the IUI procedure, DNA fragmentation index (DFI), sperm morphology, and other clinical parameters related to male infertility, such as smoking history or varicocele. Therefore, the probability of post-IUI pregnancy obtained from the scoring system and the application should be approached with caution.
There is a relationship between the male partner's BMI, the total number of motile sperm after sperm preparation, the female partner's age, AMH levels, endometrial thickness, and prognostic factors from both the male and female sides with pregnancy after IUI. There is no relationship between the male partner's age, sperm preparation method, female partner's BMI, female infertility etiology, type of stimulation, and the size of the mature follicle post-stimulation with pregnancy after IUI. We suggest that in daily practice, significant variables such as male BMI, female age, TPSC, AMH levels, and endometrial thickness are factors that need to be corrected before IUI to support therapeutic success. The path analysis approach is one method that can be recommended for future research to assess the relationship of each variable in supporting the success of IUI.
What is known about this topic
- Intrauterine Insemination (IUI) was selected as a treatment for infertility due to the simple method, affordability, and non-invasive procedure other than IVF procedure;
- Success rate IUI considerably raised if this couple had minimal etiology factors for their infertility;
- Lack of information about pregnancy programs for couples affects the decreased success rate of pregnancy programs.
What this study adds
- This study reports the scoring in the Indonesian population by including two variables, total progressive sperm count (TPSC) and endometrial thickness, which were not previously reported in other studies;
- Our study showed different results, such as the first insemination attempt having a greater pregnancy chance than the second or third attempt.
The authors declare no competing interests.
Geraldo Laurus: methodology, writing-original draft, data curation, data analysis, writing-review, and editing. Agustinus Agustinus: supervision, methodology, writing review, and editing. Cennikon Pakpahan: conceptualization, methodology, data analysis, writing-review and editing. Vellyana Lie: writing-review and editing. Maitra Djiang Wen: writing-review and editing. All the authors read and approved the final version of this manuscript.
We thank the medical and administrative team at Bocah Indonesia Fertility Clinic and SMART IVF Anna Hospital for supporting this study.
Table 1: participants' characteristics
Table 2: relationship between prognostic factors and pregnancy
Table 3: results of multivariate analysis of prognostic factors for post-intrauterine insemination pregnancy
Table 4: scoring results of post-intrauterine insemination pregnancy prognostic factors
Table 5: total difference test for post-intrauterine insemination pregnancy prognostic factor scores
Figure 1: analysis framework in the study
Figure 2: receiver operating characteristic analysis of male and female factors; A) male age; B) male body mass index; C) female age; D) female body mass index; E) total motility sperm count; F) total progressive sperm count; G) female anti-mullerian hormone levels; H) numbers of mature follicles; I) size of mature follicles; J) endometrium thickness
- Abdelkader AM, Yeh J. The Potential Use of Intrauterine Insemination as a Basic Option for Infertility: A Review for Technology-Limited Medical Settings. Obstet Gynecol Int. Obstet Gynecol Int. 2009:2009:584837. PubMed | Google Scholar
- Lee RK, Hou JW, Ho HY, Hwu YM, Lin MH, Tsai YC et al. Sperm morphology analysis using strict criteria as a prognostic factor in intrauterine insemination. Int J Androl. 2002 Oct;25(5):277-80 PubMed | Google Scholar
- Fuentes Ávila A, Blasco Sanz R, Cortés Alaguero C. Effect of Sperm Morphology in Intrauterine Insemination: Analysis of 115 Cycles and Literature Review. Obstet Gynecol Surv. 2021 Mar;76(3):170-4. PubMed | Google Scholar
- Soria M, Pradillo G, García J, Ramón P, Castillo A, Jordana C et al. Pregnancy Predictors after Intrauterine Insemination: Analysis of 3012 Cycles in 1201 Couples. J Reprod Infertil. 2012 Jul;13(3):158-66. PubMed | Google Scholar
- Dinelli L, Courbière B, Achard V, Jouve E, Deveze C, Gnisci A et al. Prognosis factors of pregnancy after intrauterine insemination with the husband´s sperm: Conclusions of an analysis of 2,019 cycles. Fertil Steril. 2014;101(4):994-1000. PubMed | Google Scholar
- Ferraretti AP, Goossens V, Kupka M, Bhattacharya S, De Mouzon J, Castilla JA et al. Assisted reproductive technology in Europe, 2009: Results generated from European registers by ESHRE. Human Reproduction. 2013;28(9):2318-31. PubMed | Google Scholar
- Gubert PG, Pudwell J, Van Vugt D, Reid RL, Velez MP. Number of motile spermatozoa inseminated and pregnancy outcomes in intrauterine insemination. Fertil Res Pract. 2019 Sep 2:5:10. PubMed | Google Scholar
- Michau A, El Hachem H, Galey J, Le Parco S, Perdigao S, Guthauser B et al. Predictive factors for pregnancy after controlled ovarian stimulation and intrauterine insemination: A retrospective analysis of 4146 cycles. J Gynecol Obstet Hum Reprod. 2019 Dec 1;48(10):811-5. PubMed | Google Scholar
- Vargas-Tominaga L, Alarcón F, Vargas A, Bernal G, Medina A, Polo Z. Associated factors to pregnancy in intrauterine insemination. J Bras Reprod Assist. 2020;24(1):66-9. PubMed | Google Scholar
- De Mouzon J, Goossens V, Bhattacharya S, Castilla JA, Ferraretti AP, Korsak V et al. Assisted reproductive technology in Europe, 2007: Results generated from European registers by ESHRE. Human Reproduction. 2012;27(4):954-66. PubMed | Google Scholar
- Mathieu C, Ecochard R, Bied V, Lornage J, Czyba JC. Cumulative conception rate following intrauterine artificial insemination with husband´s spermatozoa: influence of husband´s age. Hum Reprod. 1995 May;10(5):1090-7. PubMed | Google Scholar
- Muthigi A, Jahandideh S, Bishop LA, Naeemi FK, Shipley SK, O´Brien JE et al. Clarifying the relationship between total motile sperm counts and intrauterine insemination pregnancy rates. In: Fertility and Sterility. Fertil Steril. 2021 Jun;115(6):1454-1460. PubMed | Google Scholar
- Sicchieri F, Silva AB, e Silva ACJ de SR, Navarro PA de AS, Ferriani RA, Dos Reis RM. Prognostic factors in intrauterine insemination cycles. JBRA Assist Reprod. 2018 Mar 1;22(1):2-7. PubMed | Google Scholar
- Huniadi A, Bimbo-Szuhai E, Botea M, Zaha I, Beiusanu C, Pallag A et al. Fertility Predictors in Intrauterine Insemination (IUI). J Pers Med. 2023 Feb 23;13(3):395. PubMed | Google Scholar
- Merviel P, Heraud MH, Grenier N, Lourdel E, Sanguinet P, Copin H. Predictive factors for pregnancy after intrauterine insemination (IUI): An analysis of 1038 cycles and a review of the literature. Fertil Steril. 2010 Jan 1;93(1):79-88. PubMed | Google Scholar
- Kamath MS, Bhave PTK, Aleyamma TK, Nair R, Chandy A, Mangalaraj AM et al. Predictive factors for pregnancy after intrauterine insemination: A prospective study of factors affecting outcome. J Hum Reprod Sci. 2010 Sep;3(3):129-34. PubMed | Google Scholar
- Khan SN, Storer BL, Peck JD, Goddard LD, Hansen KR, Craig LB. Does a difficult intrauterine insemination lower pregnancy rates and live birth rates? Fertil Steril. 2013 Sep;100(3):S378. Google Scholar
- Ayas S, Gurbuz A, Ayaz R, Reşit Asoglu M, Selcuk S, Alkan A et al. Efficacy of passive uterine straightening during intrauterine insemination on pregnancy rates and ease of technique. Journal of Obstetrics and Gynaecology Research. 2012 Jan;38(1):291-6. PubMed | Google Scholar
- Green KA, Zolton JR, Schermerhorn SMV, Lewis TD, Healy MW, Terry N et al. Progesterone luteal support after ovulation induction and intrauterine insemination: an updated systematic review and meta-analysis. Fertil Steril. 2017 Apr 1;107(4):924-933.e5. PubMed | Google Scholar
- Luo Y, Wu S, Yuan J, Zhou H, Zhong Y, Zhang M et al. Evaluation of Prognostic Factors for Clinical Pregnancy Rate Following Artificial Insemination by Husband in the Chinese Population. Front Med (Lausanne). 2021 May 10:8:638560. PubMed | Google Scholar
- Zippl AL, Wachter A, Rockenschaub P, Toth B, Seeber B. Predicting success of intrauterine insemination using a clinically based scoring system. Arch Gynecol Obstet. 2022 Nov 1;306(5):1777-86. PubMed | Google Scholar
- Jayakrishnan K, Sneha Ann A, Divya N. Factors affecting success of intrauterine insemination: A 3 year prospective study. Int J Reprod Contracept Obstet Gynecol. 2016 Apr 1;5(4):1077-83. Google Scholar
- Ye F, Cao W, Lin J, Du Y, Lan L, Dong Y et al. The pregnancy outcomes of intrauterine insemination with husband´s sperm in natural cycles versus ovulation stimulated cycles: A retrospective study. Biosci Trends. 2018;12(5):463-9. PubMed | Google Scholar
- Dommaraju P, Tan J. Going against global marriage trends: the declining age at first marriage in Indonesia. Asian Population Studies. 2024 May 3;20(2):144-64. Google Scholar
- Bennett LR, Wiweko B, Hinting A, Adnyana IP, Pangestu M. Indonesian infertility patients´ health seeking behaviour and patterns of access to biomedical infertility care: An interviewer administered survey conducted in three clinics. Reprod Health. 2012 Sep 28:9:24. PubMed | Google Scholar
- Hartati TS, Isaura ER. Three Body Mass Index Classification Comparison In Predicting Hypertension Among Middle-Aged Indonesians. National Nutrition Journal/Media Gizi Indonesia. 2023 Jan 1;18(1). Google Scholar
- Haththotuwa RN, Wijeyaratne CN, Senarath U. Worldwide epidemic of obesity. In: Obesity and Obstetrics. Elsevier. 2020;3-8. Google Scholar
- Kim H, Subramanian V, Baird F, Beebeejaun Y, Sarris I, Kamath MS et al. Effect of female body mass index on intrauterine insemination outcomes: a systematic review and meta-analysis. Hum Fertil. 2023;26(6):1511-8. PubMed | Google Scholar
- Starosta A, Gordon CE, Hornstein MD. Predictive factors for intrauterine insemination outcomes: a review. Fertil Res Pract. 2020 Dec;6(1). Google Scholar
- Genchi VA, Rossi E, Lauriola C, D´Oria R, Palma G, Borrelli A et al. Adipose Tissue Dysfunction and Obesity-Related Male Hypogonadism. Int J Mol Sci. 2022 Jul 25;23(15):8194. PubMed | Google Scholar
- Chaudhuri GR, Das A, Kesh SB, Bhattacharya K, Dutta S, Sengupta P et al. Obesity and male infertility: multifaceted reproductive disruption. Middle East Fertility Society Journal. 2022 Apr 4;27(1):8. Google Scholar
- Stalzer A, Seybold D, Gantt P, Broce M, Cronkright A. Anti-Müllerian Hormone: A Predictor of Successful Intrauterine Insemination. Cureus. 2023 Oct 17;15(10):e47200. PubMed | Google Scholar
- Demir B, Dilbaz B, Cinar O, Karadag B, Tasci Y, Kocak M et al. Factors affecting pregnancy outcome of intrauterine insemination cycles in couples with favourable female characteristics. J Obstet Gynaecol (Lahore). 2011 Jul;31(5):420-3. PubMed | Google Scholar
- Awtani M, Mathur K, Shah S, Banker M. Infertility stress in couples undergoing intrauterine insemination and in vitro fertilization treatments. J Hum Reprod Sci. 2017 Jul 1;10(3):221-5. PubMed | Google Scholar
- Ombelet W. Evidence-based recommendations for IUI in daily practice. Middle East Fertility Society Journal. 2013 Jun 1;18(2):74-7. Google Scholar
- Allahbadia GN, Ata B, Lindheim SR, Woodward BJ, Bhagavath B, editors. Textbook of assisted reproduction. Springer; 2020 Aug 5. Google Scholar
- Lei R, Chen S, Li W. Advances in the study of the correlation between insulin resistance and infertility. Front Endocrinol (Lausanne). 2024 Jan 26:15:1288326. PubMed | Google Scholar
- Zako A, Martynowicz I, Citko A, Konopka P, Paszko A, Pawowski M et al. The Influence of Lifestyle on Male Fertility in the Context of Insulin Resistance-Identification of Factors That Influence Semen Quality. J Clin Med. 2024 May 9;13(10):2797. PubMed | Google Scholar
- Stavros S, Potiris A, Molopodi E, Mavrogianni D, Zikopoulos A, Louis K et al. Sperm DNA Fragmentation: Unraveling Its Imperative Impact on Male Infertility Based on Recent Evidence. Int J Mol Sci. 2024 Sep 22;25(18):10167. PubMed | Google Scholar
Search
This article authors
On Pubmed
On Google Scholar
Citation [Download]
Navigate this article
Similar articles in
Key words
Tables and figures
 Figure 2: receiver operating characteristic analysis of male and female factors; A) male age; B) male body mass index; C) female age; D) female body mass index; E) total motility sperm count; F) total progressive sperm count; G) female anti-mullerian hormone levels; H) numbers of mature follicles; I) size of mature follicles; J) endometrium thickness
Figure 2: receiver operating characteristic analysis of male and female factors; A) male age; B) male body mass index; C) female age; D) female body mass index; E) total motility sperm count; F) total progressive sperm count; G) female anti-mullerian hormone levels; H) numbers of mature follicles; I) size of mature follicles; J) endometrium thickness