Factors associated with pelvic organ prolapse: case-control study in two hospitals of Bon-Berger and Saint Georges of the city of Kananga in the Democratic Republic of the Congo
Antoine Tshimbundu Kayembe, Patrick Kahindo Muyayalo, Andy Mbangama Muela, Rahma Raschid Tozin
Corresponding author: Antoine Tshimbundu Kayembe, Department of Gynaecology and Obstetrics, Faculty of Medicine, University Notre-Dame of Kasayi, Central Kasaï, Democratic Republic of the Congo 
Received: 08 Apr 2024 - Accepted: 02 Jun 2024 - Published: 27 Jun 2024
Domain: Gynecology,Obstetrics and gynecology
Keywords: Factors associated, pelvic organs prolapse, Bon-Berger Hospital, Saint Georges Hospital, Kananga, DR Congo
©Antoine Tshimbundu Kayembe et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Antoine Tshimbundu Kayembe et al. Factors associated with pelvic organ prolapse: case-control study in two hospitals of Bon-Berger and Saint Georges of the city of Kananga in the Democratic Republic of the Congo. Pan African Medical Journal. 2024;48:76. [doi: 10.11604/pamj.2024.48.76.43545]
Available online at: https://www.panafrican-med-journal.com//content/article/48/76/full
Research 
Factors associated with pelvic organ prolapse: case-control study in two hospitals of Bon-Berger and Saint Georges of the city of Kananga in the Democratic Republic of the Congo
Factors associated with pelvic organ prolapse: case-control study in two hospitals of Bon-Berger and Saint Georges of the city of Kananga in the Democratic Republic of the Congo
![]() Antoine Tshimbundu Kayembe1,2,3,&,
Antoine Tshimbundu Kayembe1,2,3,&, ![]() Patrick Kahindo Muyayalo4, Andy Mbangama Muela4, Rahma Raschid Tozin4
Patrick Kahindo Muyayalo4, Andy Mbangama Muela4, Rahma Raschid Tozin4
&Corresponding author
Introduction: pelvic organ prolapse is a disease or disorder of the pelvic floor that can both worsen or regress, especially in the postpartum period. It carries a high risk of recurrence after surgical treatment. The objective of this study is to identify the factors associated with pelvic organ prolapse in the two hospitals of Bon-Berger and Saint-Georges in the town of Kananga in the Democratic Republic of Congo.
Methods: this is a case-control study that is carried out on the medical records of 134 patients admitted to the gynecology departments of the Bon-Berger Hospitals of Tshikaji and Saint Georges of Katoka, from January 1st to July 31st, 2023 and based on non-probability convenience sampling for case selection. The ANOVA test, Chi-test and logistic regression with adjustment are used in the statistical analyses.
Results: the factors associated with the occurrence of pelvic organs prolapse are heavy physical work (aOR: 4.031, 95% CI: 2.760-9.212; p: 0.004), malnutrition in the form of BMI less than 18.5 (aOR: 2.550, 95% CI: 1.360-5.840; p: 0.023), multiparity (aOR: 1.520, 95% CI: 1.234-4.320; p: 0.015), vaginal delivery (aOR: 3.020, 95% CI: 0.063-14.470; 0.002), fetal macrosomia (aOR: 4.290, 95% CI: 3.320-5.550; p: 0.032), pelvic tears (aOR: 2.910, 95% CI: 2.090-5.930, p: 0.006) and menopause (aOR: 3.110, 95% CI: 1.040-9.250, p: 0.001).
Conclusion: these results can serve as a basis for screening women at high risk of suffering from pelvic organ prolapse during gynecological and obstetrical consultations and for in-depth studies seeking the matrix metalloproteinases associated with pelvic organ prolapse to improve its treatment in hospitals of our town of Kananga.
Pelvic organ prolapse is defined as a permanent or strained protrusion into or outside the vaginal canal, vulvar opening, and pelvic organs such as the uterus, bladder, rectum and small intestine [1,2]. Although it is not fatal, genital prolapse has a significant impact on the quality of life of patients and leads to serious social problems including loss of self-esteem [1-7]. According to the WHO, the pelvic organ prolapse affects around 50% of women who have given birth and its lifetime prevalence varies from 20 to 50%; which is one of the most common reasons for gynecological surgery [2-5]. Previous studies have estimated that a woman's lifetime risk of having at least one surgery for pelvic organ prolapse and urinary incontinence is 11.1%, with a 10-year reoperation rate of 17% [2,8,9].
Pelvic organ prolapse is a major cause of morbidity among women in both high- and low-income countries. The prevalence of pelvic organ prolapse varies from 2.90 to 97.70% worldwide depending on the study methods used. It is estimated from 2.90 to 11.40% when the method used is a symptom questionnaire [10-19] and from 31.80 to 97.70% when a clinical examination is carried out with pelvic organ prolapse quantification (POPQ) [8,20-28]. The global prevalence of pelvic organ prolapse has been reported to be around 9.00% and this figure is however estimated to be closer to 20.00% in low-income countries [29]. In sub-Saharan Africa, studies conducted in Ghana, Gambia, Ethiopia and Tanzania have reported prevalence rates ranging from 12 to 65.00% [30-33]. This prevalence is 24.12% in the town of Kananga in the Democratic Republic of Congo [34].
The risk factors involved in the occurrence of pelvic organs prolapse are known and divided into the following two groups: modifiable risk factors such as obesity, malnutrition, vaginal delivery, parity, smoking, fetal macrosomia, perineal tears, carrying heavy objects, low socio-economic level [1,35-39], anemia and situations of oxidative stress and cellular apoptosis in connective tissues [40-43]; and non-modifiable risk factors such as age, race including white race, menopause, chronic obstructive pulmonary disease (COPD), spinal curvature abnormalities (thoracic kyphosis, loss of lumbar lordosis), history family and personal genital prolapse, previous pelvic surgeries (hysterectomy) and chronic constipation [1,35-39,44,45].
The lack of data on factors associated with pelvic organ prolapse in our town of Kananga justifies the present study, the objective of which is to identify factors associated with pelvic organ prolapse in the two hospitals of Bon-Berger and Saint-Georges in the town of Kananga in the Democratic Republic of Congo.
Study design and setting: this is a case-control study in which the cases consist of patients suffering from pelvic organ prolapse and the controls consist of patients suffering from other gynecological pathologies recorded during the mass campaign organized in the gynecological departments of two General reference hospitals from the city of Kananga: Bon-Berger Hospital in Tshikaji and Saint George Hospital in Katoka, from January 1st to July 31st, 2023. These two hospitals were chosen because of the presence of trained and experienced medical staff, the high attendance of patients suffering from pelvic organ prolapse, and more or less free treatment of pelvic organ prolapse through the various mass campaigns in the fistula cure account. Therefore, these 2 hospitals constitute references for the treatment of pelvic organ prolapse in the city of Kananga.
Study population: we used the medical records of patients aged between 30 and 79 years who suffered from genital prolapse (cases) and other gynecological pathologies (controls) and were treated during the mass campaign in the gynecology departments of hospitals of Bon-Berger of Tshikaji and Saint George of Katoka in the town of Kananga from January 1st to July 31st, 2023, and matched according to age. Our sampling is non-probabilistic for convenience. The following criteria allowed us to include the patients in the study: patients aged between 30 and 79 years, suffering from genital prolapse, treated during the mass campaign in the gynecology departments of hospitals in Bon-Berger of Tshikaji and Saint George of Katoka in the town of Kananga from January 1st to July 31st, 2023 and whose medical file was complete. Patients who did not meet these inclusion criteria and incomplete medical records (those containing less than 50% of variables studied) or were not found were excluded. Our sample size was calculated using the following formula:
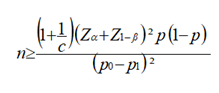
and T= n x c [36,37,39] Where:
n = sample size
P0 = Controls proportion (0.0116)
P1= Cases proportion (0.105)
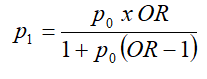 ;
;
P= proportion in the two groups (0.058)
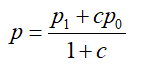
c = number of controls by cases (c=1)
T= number of controls
Zα = value of Z for the risk of the first kind (1.645)
α = the risk of type I error (0.05)
Z1-β= value of Z corresponding to a surface equal to the power of the test (1-β). The latter constitutes the probability of finding a significant difference (1.282). (1-β) = the desired power (0.9).
OR = minimum OR that we set for the study to be of public health interest and estimated at 10 based on studies on the Visco and Yuan model [46].
The calculated sample size is greater than 107 cases. One control will be matched to one case (c=1) and the matching criterion is the age of the patients because the latter is a confounding factor and meets the matching criteria.
We excluded all medical records not found and those containing less than 50% of the variables studied. One hundred and thirty-four cases and 134 controls were included and 4 cases were excluded because the files were not found (i.e. a total of 138 cases of pelvic organ prolapse recorded).
Data collection: data were collected from registers of gynecology departments, those of the operating room, medical files of patients in gynecology departments of two hospitals and the data collection record. The variables of our study are: patient age, weight, height, BMI, parity, pelvic surgery, vaginal deliveries and their number, fetal macrosomia, pelvic tears, menopause, family history of prolapse genital prolapse, personal history of pelvic organs prolapse, smoking and spinal abnormalities. The data collection was done as follows: we first identified the names of patients who had suffered from pelvic organ prolapse in the operating room and gynecology registers, then searched medical records based on the names of identified patients and finally transcribed the data from the medical records of these patients identified in the data collection sheet.
Definitions:
Parity: number of pregnancies that reached 28 weeks of amenorrhea in a woman [39].
Primiparity: presence or history of pregnancy that reached 28 weeks of amenorrhea in a woman [39].
Pauciparity: the presence of two or three pregnancies that reached 28 weeks of amenorrhea in a woman [39].
Multiparity: the story of more than four pregnancies reaching 28 weeks of amenorrhea in one woman [39].
Body mass index (BMI): ratio of weight expressed in kilograms and height in meters squared [39].
Malnutrition: this corresponds to a body weight of less than 50kg or a body mass index of less than 18.5kg/m² [39].
Statistical analysis: data were analyzed using Statistical Package for Social Sciences (SPSS) software version 29. The ANOVA test was used to perform the intergroup comparison of means, the chi-square test to perform the intergroup comparison of proportions, the univariable logistic regression to evaluate the strength of association between risk factors and the occurrence of pelvic organs prolapse and multivariable logistic regression to identify the most determining factors of pelvic organs prolapse. The statistical significance threshold for our results is set at the value of p < 0.05. The variables that were statistically significantly associated with the occurrence of pelvic organ prolapse in the Anova test and chi-square test were included in the univariable model while those which were significantly associated with the occurrence of pelvic organ prolapse in the univariable model were included in the multivariable model.
Ethical considerations: principles of medical ethics and documentary studies rules were respected; data were collected confidentially and treated anonymously. Our study obtained authorization from the Ethics Committee of the Kinshasa Health School and the local committee of different hospitals. The reference number of the approval by the Ethics Committee is N°ESP/CE/19/2023.
The risk factors whose proportion was found to be higher, in a statistically significant manner, in the group of cases compared to that of the controls are heavy physical work, malnutrition 1 with body weight <50Kg, malnutrition 2 with BMI <18.50Kg/m², multiparity, vaginal delivery, number of vaginal deliveries ≥4, fetal macrosomia, pelvic tears and menopause (Table 1).
Univariable analysis allowed us to note a significant association between the occurrence of pelvic organs prolapse and the following risk factors: heavy physical work, malnutrition in the form of body weight less than 50Kg and BMI less than 18.5, multiparity, vaginal delivery, number of vaginal deliveries greater than or equal to 4, fetal macrosomia, pelvic tear and menopause in our environment (Table 2).
According to multivariable analysis, heavy physical work (aOR: 4.031, 95% CI: 2.760-9.212; p: 0.004), malnutrition in the form of BMI less than 18.5 (aOR: 2.550, 95% CI: 1.360-5.840; p: 0.023), multiparity (aOR: 1.520, 95% CI: 1.234-4.320; p: 0.015), vaginal delivery (aOR: 3.020, 95% CI: 0.063-14.470; 0.002), fetal macrosomia (aOR: 4.290, 95% CI: 3.320-5.550; p: 0.032), pelvic tears (aOR: 2.910, 95% CI: 2.090-5.930, p: 0.006) and menopause (aOR: 3.110, 95% CI: 1.040-9.250, p: 0.001) are the independent factors associated with pelvic organs prolapse in the town of Kananga (Table 2).
This study aimed to identify factors associated with pelvic organs prolapse in the two hospitals of Bon-Berger and Saint-Georges in the town of Kananga in the Democratic Republic of Congo and recorded as non-molecular factors associated with pelvic organ prolapse: heavy physical work, malnutrition in the form of BMI less than 18.5, multiparity, vaginal delivery, fetal macrosomia, pelvic tears and menopause.
Heavy physical labor in the form of heavy lifting and agriculture is a factor associated with pelvic organ prolapse in our case series. It significantly increases the risk of pelvic organ prolapse occurring by 7.5. Our observations corroborate those of Masenga et al. in Tanzania [33], by Megabiaw et al. in Ethiopia [32], Scherf et al. in Gambia [30] and Wusu-Ansah et al. in Ghana [31]. The relationship between light physical work and the occurrence of pelvic organ prolapse is based on the increase, through this work, in intra-abdominal pressure, reaching 10.0±0.6 mmHg, at the basis of the loss of organs outside the pelvis and ligamentous lesions activate matrix metalloproteinases and collagen degradation [38,47,48].
Malnutrition is also a factor associated with pelvic organ prolapse in our city and significantly increases the risk of pelvic organ prolapse occurring by 2 in the form of body weight less than 50Kg and by 3 in the form of BMI less than 18.50Kg/m². Our results are consistent with those of many other authors in the literature [29,30,32,33]. The occurrence of pelvic organ prolapse in malnourished women is secondary to the stimulation of massive proteolysis with a view to gluconeogenesis, this proteolysis also concerns collagen, the degradation of which leads to the loss of collagen at the pelvic ligament level, ligamentous hyperlaxity and the decline resistance to ligament traction, sources of pelvic organs prolapse [48].
Multiparity (parity ≥4) is also a factor associated with pelvic organ prolapse. It significantly increases the risk of pelvic organ prolapse occurring by 2. Our finding is in agreement, not only with that of Tshimbundu et al. in Kinshasa who showed that parity ≥4 is a risk factor for pelvic organ prolapse [39], but also with that of many other authors [30,36,37,49,50]. The occurrence of pelvic organs prolapse in pares ≥4 is due to the increase, linked to this parity ≥4, in the risk of pudendal nerve damage (stretching and compression) and direct trauma to the pelvic floor muscles (pubococcygeus, levator ani and anal sphincter) [47,51,52]. This leads to the defect of the pelvic floor, the site of stimulation of the activity of matrix metalloproteinases, the degradation of collagen and the reduction of collagen and tissue resistance as well as the source of occurrence of prolapse [48,53].
Like other studies [30,37,47,49,50,54], our study also showed that vaginal delivery, number of vaginal deliveries ≥4 and perineal tears are factors associated with pelvic organ prolapse. The risk of prolapse occurring was significantly increased by 7 in the case of vaginal deliveries, by 2 in the case of several vaginal deliveries ≥4 and by 8 in the case of perineal tears. Our results meet, on the one hand, those of Kayembe et al. in Kinshasa [39] and on the other hand those of many other authors in the literature [36,47,49]. The mechanism of occurrence of pelvic organs prolapse in cases of vaginal delivery and perineal tears is based on the increased risk of pudendal nerve damage and deterioration of the posterior level thanks to damage to the central fibrous core of the perineum and anal sphincters. These lesions activate matrix metalloproteinases to cause local collagen degradation, leading to the reduction of collagen which weakens the pelvic floor, the source of pelvic organ prolapse [47,48,51-53].
Fetal macrosomia is a factor associated with pelvic organ prolapse and increases the risk of pelvic organ prolapse occurring by 5 in our city of Kananga. Our results agree on the one hand with those of Thubert et al. in Kinshasa [38] and on the other hand those of many other authors in the literature where fetal macrosomia was also significantly associated with the occurrence of pelvic organ prolapse [30,36-38,47,49,50,52]. The occurrence of pelvic organ prolapse and fetal macrosomia are linked by the severity of pelvic floor lesions, secondary to the delivery of macrosomal newborns [38,47]. These lesions also stimulate the activity of matrix metalloproteinases which are the basis of collagen degradation, collagen reduction and tissue resistance, a source of pelvic organ prolapse [47,48,51-53].
Menopause is a factor associated with pelvic organ prolapse and significantly triples the risk of prolapse occurring. Our results are similar not only to those of Kayembe et al. in Kinshasa [39] but also to those of many authors in literature [30,47,50,54]. The role of menopause in the occurrence of pelvic organs prolapse is based on post-menopausal estrogen insufficiency which leads to changes in vaginal trophism, tissue cellularity and collagen metabolism (by stimulating the degradation of collagen by matrix metalloproteinases at the basis of collagen reduction) [47,48,55,56]. Estrogen receptors have been identified in the bladder trigone, urethra, vaginal mucosa, perineal tendinous arch, uterosacral ligaments and levator ani [47,52,55,56]. Hence the reduction in estrogen intake leads to atrophy of all of these tissues, responsible for the weakness of the pelvic floor which is the basis of the occurrence of pelvic organs prolapse [47,52,55,56].
The independent factors associated with pelvic organ prolapse in our environment are heavy physical work, malnutrition (BMI <18.5), multiparity, vaginal delivery of fetal macrosomia, complicated by pelvic tears and menopause. Our results corroborate those of other authors in the literature [30,36,38,47,49-52,55,56]. The profile of the patient suffering from pelvic organs prolapse in the town of Kananga is a malnourished, multiparous menopause with a history of vaginal delivery of a macrosome baby, complicated by perineal tears and performing heavy physical work while that of the patient suffering from pelvic organs prolapse in Kinshasa is an obese menopausal woman with a history of vaginal delivery of macrosomia, complicated by perineal tear [39]. The difference between the two profiles in the same country (DR Congo) can be explained by the fact that most patients in the town of Kananga come from surrounding rural areas where they are subjected to heavy physical work (country, etc.) for survival and that the town of Kananga is one of the areas where the deadly Kamuina-nsapu conflicts took place (commonly called the "Kamuina-nsapu phenomenon") in 2017 with their consequences including famine and malnutrition on the local population who are struggling to survive or to emerge despite the multiple local recovery plans organized by the national government and non-governmental organizations. Hence the need to monitor and strengthen these recovery plans already established. The consideration of multiparity as a criterion for matching controls to cases in the Kinshasa study made it disappear from the profile of women suffering from pelvic organ prolapse in Kinshasa after the calculation of the multivariate logistic regression [39]. This was not the case in this study.
Our data can serve not only as a basis for screening women at high risk of suffering from pelvic organs prolapse during gynecological and obstetrical consultations to improve treatment but also as a base for more in-depth studies seeking the molecular factors associated with pelvic organs prolapse such as matrix metalloproteinases associated with pelvic organ prolapse in our town of Kananga because according to literature, all our factors associated with pelvic organs prolapse (heavy physical work, malnutrition in the form of BMI less than 18.5, multiparity, vaginal delivery, fetal macrosomia, pelvic tears and menopause) activate or stimulate the activity of matrix metalloproteinases which degrade the collagens at the base of decrease in collagen content at the pelvic level, source of pelvic ligament hyperlaxity and of pelvic organs prolapse [40,48,52,53]. In short, all our factors above would go through the increase in matrix metalloproteinases to cause pelvic organ prolapse.
The weakness of our study is the failure to take into account molecular factors associated with the occurrence of pelvic organ prolapse and its strength is to be the first study of risk factors for pelvic organ prolapse in hospital environments in the city of Kananga in the Democratic Republic of the Congo.
Factors associated with the occurrence of pelvic organ prolapse are heavy physical work, malnutrition (BMI <18.5), multiparity, vaginal delivery, fetal macrosomia, pelvic tears and menopause. Our results can serve not only as a basis for screening women at high risk of suffering from pelvic organ prolapse during gynecological and obstetrical consultations but also as a basis for more in-depth studies seeking the matrix metalloproteinases associated with pelvic organ prolapse to improve its management in our town of Kananga.
What is known about this topic
- The pelvic organ prolapse is a dynamic disease that can worsen or recede above all in pregnant women during the postpartum period;
- It comprises a great recurrence risk after surgical treatment and it causes several troubles (urinary, digestive and genital) that hamper the quality of life of patients;
- The lack of data on factors associated with pelvic organ prolapse in hospitals in the city of Kananga, in the DR Congo.
What this study adds
- Factors associated with pelvic organ prolapse are heavy physical work, malnutrition (BMI<18.5), multiparity, vaginal delivery, fetal macrosomia, pelvic tears and menopause in our town;
- Our results can be used in the screening of women at high risk of suffering from pelvic organ prolapse during gynecological consultations in Kananga hospitals;
- Our results can serve as a basis for in-depth studies seeking the matrix metalloproteinases associated with pelvic organ prolapse to improve its management in our city.
The authors declare no competing interests.
Conception, study design and data analysis and interpretation were handled by Antoine Tshimbundu Kayembe, Patrick Kahindo Muyayalo, Andy Mbangama Muela and Rahma Raschid Tozin. Data collection, manuscript revision and guarantor of the study is Antoine Tshimbundu Kayembe. All authors read and approved the final version of the manuscript.
We acknowledge the medical staff of Bon-Berger and Saint Georges Hospital for having allowed us and facilitated us in collecting data for this study.
Table 1: factors associated with pelvic organ prolapse
Table 2: factors independently associated with pelvic organ prolapse
- Lousquy R, Costa P, Delmas V, Haab F. Etat de lieux de l´épidémiologie des prolapsus génitaux. Progrès en Urol. 2009;19(13):907-15. PubMed | Google Scholar
- Feng Y, Wang Y, Yan B, Li L, Deng Y. Matrix Metalloproteinase-1 Expression in Women With and Without Pelvic Organ Prolapse:A Systematic Review and Meta-analysis. Clin Transl Sci. 2016;9(5):267-273. PubMed | Google Scholar
- Weber AM, Richter HE. Pelvic organ prolapse. Obstet Gynecol. 2005;106(3):615-34. PubMed | Google Scholar
- Buller JL, Thompson JR, Cundiff GW, Krueger Sullivan L, Schön Ybarra MA, Bent AE. Uterosacral ligament: description of anatomic relationships to optimize surgical safety. Obstet. Gynecol. 2001;97(6):873-9. PubMed | Google Scholar
- Subak LL, Waetjen LE, van den Eeden S, Thom DH, Vittinghoff E, Brown JS. Cost of pelvic organ prolapse surgery in the United States. Obstet Gynecol. 2001;98(4):646-51. PubMed | Google Scholar
- Gong R, Xia Z. Collagen changes in pelvic support tissues in women with pelvic organ prolapse. J Euro Obst, Gyn and Biol Reprod. 2019;234:184-9. PubMed | Google Scholar
- Lakeman MM, van der Vaart CH, Laan E, Roovers JP. The effect of prolapse surgery on vaginal sensibility. J Sex Med. 2011;8(4):1239-1245. PubMed | Google Scholar
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501-6. PubMed | Google Scholar
- Denman MA, Gregory WT, Boyles SH, Smith V, Edwards SR, Clark AL. Reoperation 10 years after surgically managed pelvic organ prolapse and urinary incontinence. Am J Obstet Gynecol. 2008;198(5):555.e1-5. PubMed | Google Scholar
- Bradley CS, Zimmerman MB, Wang Q, Nygaard IE; Women's Health Initiative. Vaginal descent and pelvic floor symptoms in postmenopausal women: a longitudinal study. Obstet Gynecol. 2008;111(5):1148-53. PubMed | Google Scholar
- Barber MD, Kuchibhatla MN, Pieper CF, Bump RC. Psychometric evaluation of 2 comprehensive condition-specific quality of life instruments for women with pelvic floor disorders. Am J Obstet Gynecol. 2001;185(6):1388-95. PubMed | Google Scholar
- Barber MD, Neubauer NL, Klein-Olarte V. Can we screen for pelvic organ prolapse without a physical examination in epidemiologic studies? Am J Obstet Gynecol. 2006;195(4):942-8. PubMed | Google Scholar
- Eva UF, Gun W, Preben K. Prevalence of urinary and fecal incontinence and symptoms of genital prolapse in women. Acta Obstet Gynecol Scand. 2003 Mar;82(3):280-6. PubMed | Google Scholar
- MacLennan AH, Taylor AW, Wilson DH, Wilson D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. Bri J Obstet Gynecol. 2000;107(12):1460-70. PubMed | Google Scholar
- Miedel A, Tegerstedt G, Maehle-Schmidt M, Nyrén O, Hammarström M. Symptoms and pelvic support defects in specific compartments. Obstet Gynecol. 2008;112(4):851-8. PubMed | Google Scholar
- Mouritsen L. Classification and evaluation of prolapse. Best Pract Res Clin Obstet Gynaecol. 2005;19(6):895-911. PubMed | Google Scholar
- Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J et al. Prevalence of pelvic floor disorders in US women. JAMA. 2008;300(11):1311-6. PubMed | Google Scholar
- Rortveit G, Brown JS, Thom DH, Van Den Eeden SK, Creasman JM, Subak LL. Symptomatic pelvic organ prolapse: prevalence and risk factors in a population-based, racially diverse cohort. AJOG. 2007;109(6):1396-403. PubMed | Google Scholar
- Slieker-ten Hove MC, Pool-Goudzwaard AL, Eijkemans MJ, Steegers-Theunissen RP, Burger CW, Vierhout ME. Symptomatic pelvic organ prolapse and possible risk factors in a general population. Am J Obstet Gynecol. 2009;200(2):1841-7. PubMed | Google Scholar
- Handa VL, Garrett E, Hendrix S, Gold E, Robbins J. Progression and remission of pelvic organ prolapse: A longitudinal study of menopausal women. Am J Obstet Gynecol. 2004;190(1):27-32. PubMed | Google Scholar
- Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women´s Health initiative: gravity and gravidity. Am J Obstet Gynecol. 2002;186(6):1160-6. PubMed | Google Scholar
- Nygaard I, Bradley C, Brandt D; Women's Health Initiative. Pelvic organ prolapse in older women: prevalence and risk factors. Obstet Gynecol. 2004;104(3):489-97. PubMed | Google Scholar
- Samuelsson EC, Victor FT, Tibblin G, Svärdsudd KF. Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. Am J Obstet Gynecol. 1999;180(2 Pt 1):299-305. PubMed | Google Scholar
- Sewell CA, Chang E, Sultana CJ. Prevalence of genital prolapse in 3 ethnic groups. J Reprod Med. 2007;52(9):769-73. PubMed | Google Scholar
- Swift SE. The distribution of pelvic organ support in a population of female subjects seen for routine gynecologic health care. Am J Obstet Gynecol. 2000;183(2):277-85. PubMed | Google Scholar
- Trowbridge ER, Fultz NH, Patel DA, DeLancey JO, Fenner DE. Distribution of pelvic organ support measures in a population based sample of middle-aged, community-dwelling African American and white women in southeastern Michigan. AJOG. 2008;198(5):5481-6. PubMed | Google Scholar
- Blain G, Dietz HP. Symptoms of female pelvic organ prolapse: correlation with organ descent in women with single compartment prolapse. Aust N Z J Obstet Gynaecol. 2008;48(3):317-21. PubMed | Google Scholar
- Versi E, Harvey MA, Cardozo L, Brincat M, Studd JW. Urogenital prolapse and atrophy at menopause:a prevalence study. Int Urogynecol J. 2001;12(2):107-10. PubMed | Google Scholar
- Walker GJ, Gunasekera P. Pelvic organ prolapse and incontinence in developing countries: review of prevalence and risk factors. Int Urogynecol J. 2011;22(2):127-35. PubMed | Google Scholar
- Scherf C, Morison L, Fiander A, Ekpo G, Walraven G. Epidemiology of pelvic organ prolapse in rural Gambia, West Africa. BJOG. 2002;109(4):431-6. PubMed | Google Scholar
- Wusu-Ansah OK, Opare-Addo HS. Pelvic organ prolapse in rural Ghana. Int J Gynaecol Obstet. 2008;103(2):121-4. PubMed | Google Scholar
- Megabiaw B, Adefris M, Rortveit G, Degu G, Muleta M, Blystad A et al. Pelvic floor disorders among women in Dabat district, northwest Ethiopia: a pilot study. Int Urogynecol J. 2013;24(7):1135-43. PubMed | Google Scholar
- Masenga GG, Shayo BC, Rasch V. Prevalence and risk factors for pelvic organ prolapse in Kilimanjaro, Tanzania: A population based study in Tanzanian rural community. PLoS One. 2018 Apr 25;13(4):e0195910. PubMed | Google Scholar
- Kayembe AT, Ilunga BM, Muakuya JM, Muela AM, Tozin RR. Pelvic organ prolapse: a cross-sectional study during mass campaign in two hospitals in the city of Kananga in the Democratic Republic of Congo. Pan Afr Med J. 2024 Feb 8;47:52. PubMed | Google Scholar
- Chow D, Rodríguez LV. Epidemiology and prevalence of pelvic organ prolapse. Curr Opin Urol. 2013;23(4):293-8. PubMed | Google Scholar
- Chiaffarino F, Chatenoud L, Dindelli M, Meschia M, Buonaguidi A, Amicarelli F et al. Reproductive factors, family history, occupation and risk of urogenital prolapse. Eur J Obstet Gynecol Reprod Biol. 2009;82(1):63-7. PubMed | Google Scholar
- Aytan H, Ertunç D, Tok EC, Yaşa O, Nazik H Prevalence of pelvic organ prolapse and related factors in a general female population. J Turk Soc Obstet Gynecol. 2014;11(3):176-180. PubMed | Google Scholar
- Thubert T, Deffieux X, Letouzey V, Hermieu JF. Obésité et urogynécologie: revue de la littérature. Prog Urol. 2012;22(8):445-53. PubMed | Google Scholar
- Kayembe AT, Kayembe CDKK, Bebele JK, Tozin RR. Factors associated with genital prolapse at Saint-Joseph Hospital of Kinshasa. Pan Afr Med J. 2021 Dec 16;40:234. PubMed | Google Scholar
- Kim EJ, Chung N, Park SH, Lee KH, Kim SW, Kim JY et al. Involvement of oxidative stress and mitochondrial apoptosis in the pathogenesis of pelvic organ prolapse. J Urol. 2013;189(2):588-94. PubMed | Google Scholar
- Hong S, Li H, Wu D, Li B, Liu C, Guo W et al. Oxidative damage to human parametrial ligament fibroblasts induced by mechanical stress. Mol Med Rep. 2015;12(4):5342-8. PubMed | Google Scholar
- Liu C, Yang Q, Fang G, Li BS, Wu DB, Guo WJ et al. Collagen metabolic disorder induced by oxidative stress in human uterosacral ligament-derived fibroblasts: a possible pathophysiological mechanism in pelvic organ prolapse. Mol Med Rep. 2016;13(4):2999-3008. PubMed | Google Scholar
- Takacs P, Nassiri M, Gualtieri M, Candiotti K, Medina CA. Uterosacral ligament smooth muscle cell apoptosis is increased in women with uterine prolapse. Reprod Sci. 2009;16(5):447-52. PubMed | Google Scholar
- Dällenbach P, Kaelin-Gambirasio I, Dubuisson JB, Boulvain M. Risk factors for pelvic organ prolapse repair after hysterectomy. Obstet Gynecol. 2007;110(3):625-32. PubMed | Google Scholar
- Tegerstedt G, Maehle-Schmidt M, Nyrén O, Hammarström M. Prevalence of symptomatic pelvic organ prolapse in a Swedish population. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16(6):497-503. PubMed | Google Scholar
- Visco AG, Yuan L. Differential gene expression in pubococcygeus muscle from patients with pelvic organ prolapse. Am J Obstet Gynecol. 2003;189(1):102-12. PubMed | Google Scholar
- Blanc B, Deval B. (2005). Prolapsus génital: contexte nosologique et pathogénie commune. In: Pelvi-périnéologie. Springer, Paris. https://doi.org/10.1007/2-287-27807-9_19. Google Scholar
- Kieserman-Shmokler C, Swenson CW, Chen L, Desmond LM, Ashton-Miller JA, DeLancey JO. From molecular to macro: the key role of the apical ligaments in uterovaginal support. Am J Obstet & Gynecol. 2020;222(5):427-436. PubMed | Google Scholar
- Erata YE, Kilic B, Güçlü S, Saygili U, Uslu T. Risk factors for pelvic surgery. Arch Gynecol Obstet. 2002;267(1):14-8. PubMed | Google Scholar
- Ragni E, Lousquy R, Costa P, Delmas V, Haab F. Facteurs de risque et prévention des prolapsus génito-urinaires [Risk factors and prevention of genitourinary prolapse]. Prog Urol. 2009 Dec;19(13):932-8. PubMed | Google Scholar
- Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. Lancet. 2007;369(9566):1027-38. PubMed | Google Scholar
- Cnattingius S, Villamor E, Lagerros YT, Wikström AK, Granath F. High birth weight and obesity: a vicious circle across generations. Int J Obes (Lond). 2012;36(10):1320-4. PubMed | Google Scholar
- Wong MY, Harmanli OH, Agar M, Dandolu V, Grody MH. Collagen content of nonsupport tissue in pelvic organ prolapse and stress urinary incontinence. Am J Obstet Gynecol. 2003;189(6):1597-9. PubMed | Google Scholar
- Rodrigues AM, de Oliveira LM, Martins Kde F, Del Roy CA, Sartori MG, Girão MJ et al. Risk factors for genital prolapse in a Brazilian population. Urogynecological vaginal surgery service, Medicine School Paulista, federal University of São Paul, Brasil. Rev Bras Ginecol Obstet. 2009;31(1):17-21. PubMed | Google Scholar
- Jackson S, James M, Abrams P. The effect of oestradiol on vaginal collagen metabolism in postmenopausal women with genuine stress incontinence. BJOG. 2002 Mar;109(3):339-44. PubMed | Google Scholar
- Lang JH, Zhu L, Sun ZJ, Chen J. Estrogen levels and estrogen receptors in patients with stress urinary incontinence and pelvic organ prolapse. Int J Gynaecol Obstet. 2003;80(1):35-9. PubMed | Google Scholar











