Co-administration of rimonabant prevents glucose intolerance in Sprague-Dawley rats treated chronically with lopinavir/ritonavir and zidovudine: an experimental study design
Brian Lishenga Makamu, Peter Waweru Mwangi, Frederick Okonji Bukachi
Corresponding author: Brian Lishenga Makamu, Department of Medical Physiology, Egerton University, P.O Box 536, 20115 Egerton, Kenya 
Received: 15 Jan 2020 - Accepted: 03 Aug 2021 - Published: 03 May 2023
Domain: Pharmacology,Physiology,HIV prevention and care (PMTCT)
Keywords: Antiretroviral therapy, rimonabant, metabolic syndrome, hyperglycemia, endogenous cannabinoids, HIV, protease inhibitors, nucleoside reverse transcriptase inhibitors
©Brian Lishenga Makamu et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Brian Lishenga Makamu et al. Co-administration of rimonabant prevents glucose intolerance in Sprague-Dawley rats treated chronically with lopinavir/ritonavir and zidovudine: an experimental study design. Pan African Medical Journal. 2023;45:6. [doi: 10.11604/pamj.2023.45.6.21541]
Available online at: https://www.panafrican-med-journal.com//content/article/45/6/full
Research 
Co-administration of rimonabant prevents glucose intolerance in Sprague-Dawley rats treated chronically with lopinavir/ritonavir and zidovudine: an experimental study design
Co-administration of rimonabant prevents glucose intolerance in Sprague-Dawley rats treated chronically with lopinavir/ritonavir and zidovudine: an experimental study design
![]() Brian Lishenga Makamu1,&, Peter Waweru Mwangi2, Frederick Okonji Bukachi2
Brian Lishenga Makamu1,&, Peter Waweru Mwangi2, Frederick Okonji Bukachi2
&Corresponding author
Introduction: treatment of HIV infection with Protease Inhibitors (PIs) and Nucleoside Reverse Transcriptase Inhibitors (NRTIs) can lead to insulin resistance and changes in body fat distribution. Overactivity of the endogenous cannabinoid system produces similar disturbances in metabolic syndrome within the general population. However, Cannabinoid receptor type 1 antagonism, in both human and animal studies, reverses many of these biochemical and physical derangements observed in the metabolic syndrome.
Methods: using an experimental study design, fifteen adult male Sprague-Dawley rats housed under standard conditions were randomized into three groups; Control, combined Anti-Retroviral Therapy (cART) only and cART + rimonabant. Drugs were administered daily by oral gavage for four weeks. After four weeks, insulin tolerance tests were conducted, the rats were euthanised and fat depots were excised and weighed. Experimental data were analysed using STATA 16.0 with the significance level set at p<0.05. The Shapiro-Wilk test determined normalcy. In cases of significance, post hoc analysis was performed by either the Dunn test or the Tukey HSD test.
Results: Sprague Dawley rats treated with cART + Rimonabant demonstrated better insulin sensitivity (p = 0.0239) and lower body weight (p = 0.044) than rats treated with cART alone. They had leaner body composition with 58% less adiposity than cART-only rats.
Conclusion: the study results suggest a role for the endogenous cannabinoid system in cART induced metabolic derangements and physical changes. Future studies can directly assay ECS activity in cART associated metabolic syndrome.
Treatment of HIV infection with combined antiretroviral therapy (cART) regimens containing the Protease Inhibitor (PI) combination of Lopinavir/Ritonavir (LPV/r) as well as the Nucleoside Reverse Transcriptase Inhibitors (NRTIs) Zidovudine (AZT) can lead to insulin resistance along with changes in body fat distribution [1,2]. The resulting derangements, such as hyperglycaemia, hyperinsulinemia, elevated levels of soluble Tumour Necrosis Factor α, high triglyceride levels, low high-density lipoprotein cholesterol levels and low adiponectin levels, are similar to those observed in the Metabolic Syndrome among the general population [3,4]. Overactivity of the endogenous cannabinoid system (ECS) among the general population occurs in hyperglycaemic and obese states [5]. In both human and animal studies, blockage of the ECS by Cannabinoid Type 1 Receptor (CB1R) antagonists such as Rimonabant reverses many of the biochemical derangements and physical changes observed in the Metabolic Syndrome [6]. CB1R antagonism results in a reduction in body weight, serum insulin and glucose levels [7,8].
There is a high degree of convergence and similarity in the mechanisms by which antiretroviral drugs and ECS overactivity produce physical changes and metabolic derangements. Protease Inhibitors decrease pancreatic insulin secretion, bind to and block GLUT 4 as well as alter its expression in adipose tissue and reduce expression of the adipokines adiponectin and leptin [9-11]. Adiponectin resistance or a reduction in serum adiponectin levels is associated with insulin resistance. NRTIs, on the other hand, diminish whole-body glucose disposal [12]. ECS upregulation activates similar mechanisms in both animal and human studies [13,14].
In this study, we hypothesised that blocking of endogenous cannabinoid activity in the context of antiretroviral therapy would ameliorate cART induced hyperglycaemia and insulin resistance. Our study objectives were: to determine the effect of Rimonabant on insulin tolerance in LPV/r and AZT treated Sprague-Dawley rats and to determine the effect of Rimonabant on fat distribution in LPV/r and AZT treated Sprague-Dawley rats. At the end of 4 weeks, Sprague Dawley rats treated with a combination of LPV/r + AZT + Rimonabant showed increased insulin sensitivity as well as less body weight gain compared to Sprague Dawley rats treated with LPV/r + AZT only.
Chemicals used: lopinavir and ritonavir (4:1 ratio, respectively) fixed-dose combination tablets manufactured by Mylan laboratories Ltd. (Maharashtra, India). Zidovudine tablets were from Hetero drugs Ltd., (Hyderabad, India). The District Aids and STI Coordinator (DASCO), Nakuru, Kenya, donated both antiretroviral drugs. Rimonabant hydrochloride (rimonabant, 99% purity) purchased from Clearsynth Labs Limited., (Mumbai, India).
Preparation of drugs and dose calculation: a single tablet of Lopinavir/ritonavir, (200mg/50mg) was completely dissolved in 10ml of drinking water to yield a concentration of 20/5mg/ml. For Zidovudine, a single tablet (300mg) was dissolved entirely in 30mls of drinking water to yield a solution concentration of 10mg/ml. Dosages to be administered for the antiretrovirals were calculated based on the dosing guidelines for ART drugs in adult HIV patients. That is, for lopinavir/ritonavir 800 mg/200mg per day or 13.33/3.33mg/kg/day and for Zidovudine, 600mg per day, or 10mg/kg/day assuming a bodyweight of 60kg. Using body surface area normalisation, this translated to a dosage of approximately 80mg/20mg/kg/day of LPV/r and 60mg/kg/day of AZT in rats [15]. Rimonabant hydrochloride tablets were dissolved in dimethyl sulphoxide (DMSO) by gentle shaking followed by dilution with Tween 20 and saline (2% DMSO, 1% Tween 20, 97% saline) to a final concentration of 1mg/ml. It then was administered at a dose of 3mg/kg/day arrived at based on dose-response curves developed previously [16]. Rats were weighed every day, followed by the administration of individualised doses of the reconstituted drugs between 0900h and 1100h by oral gavage for four weeks.
Experimental animals: fifteen Sprague Dawley rats (Rattus norvegicus) weighing between 300 and 350 g at the beginning of the study were purchased from the Department of Biochemistry, University of Nairobi and housed in communal cages at the Department of Medical Physiology, University of Nairobi. The rats were randomized into three groups; Control, combined Anti-Retroviral Therapy (cART) only and cART + Rimonabant. The randomization scheme was generated by the Randomization website. using the method of randomly permuted blocks. The rats were also randomly selected for drug administration and experimental procedures. Sprague Dawley rats were selected because of their suitability for hormone profile studies and ease of handling. The rats were fed with standard rat chow (Unga Feeds, Nairobi, Kenya) and provided with water ad libitum. Rats were habituated to handling and testing procedures under a 12:12-h light-dark cycle (lights off at 1800 h) for two (2) weeks before testing. Dry wood shavings were used as beddings and changed daily.
The Postgraduate Research Committee, Department of Medical Physiology, School of Medicine, University of Nairobi, approved the study protocol. Rats were handled by following the U.S National Research Council Guide for the Care and Use of Laboratory Animals.
Experimental protocols
Insulin tolerance test: Sprague Dawley rats were randomised into three groups of 5 rats each. Group 1 served as the control and received a weight-matched volume of 0.5% pharmaceutical grade starch solution. Group 2 rats were treated with LPV/r + AZT while group 3 received LPV/r + AZT + Rimonabant. The insulin tolerance test (ITT) was conducted, as described by Beguinot [17]. Briefly, following a five-hour fast (at time 0), the tip of the tail was nicked with a scalpel blade. A drop of blood was expressed onto a glucose test strip and measured with a glucose meter (Prestige Smart System; Walgreens). The rats were then injected subcutaneously at the back of the neck with 0.5 units/kg of Actrapid® insulin (Novo Nordisk; U.S.A). Subsequently, blood glucose levels were measured serially at times 15, 30, 60 and 120 minutes after the insulin injection.
Bodyweight and adiposity: the rats were weighed daily before drug administration. At the end of 4 weeks, the rats were euthanised using an intraperitoneal injection of Ketamine (80mg/kg) and Xylazine (10mg/kg). Interscapular, inguinal, and epididymal fat depots were then carefully excised and weighed to measure adiposity.
Statistical analysis: experimental data were statistically analysed using STATA 16.0 statistical package with the significance level set at p<0.05. The Shapiro Wilk test determined normalcy. Normally distributed data were analysed by one way ANOVA while the Kruskal Wallis test was used to analyse non-normally distributed data. In cases of significance, posthoc analysis was performed by either the Tukey's honestly significant difference (HSD) test or the Dunn post hoc test for normally and non-normally distributed data respectively.
Insulin sensitivity: we determined insulin sensitivity by calculating the area under the curve (AUC) of the graphs obtained by plotting blood glucose concentration against time following a two-hour insulin tolerance test. Subsequently, AUC was calculated using the composite trapezoidal method by applying the pksumm and the pkexamine commands in STATA. Analysis of the AUCs found a statistically significant difference in insulin sensitivity between the treatment groups α² (2) = 6.080, p = 0.0478, with a mean rank AUC of 60.00 for the LPV/r+AZT group, 32.00 for LPV/r+AZT+Rimonabant and 28.00 for controls. A Dunn's pairwise comparison test followed, revealing that, insulin sensitivity was significantly higher in rats treated with LPV/r +AZT + Rimonabant compared to rats treated with LPV/r+AZT only (329.14 ± 85.19 vs 389.81 ± 37.96, p = 0.0238) (Table 1). There was no statistical difference in insulin sensitivity between rats treated with LPV/r+AZT+Rimonabant and control rats (p=0.39).
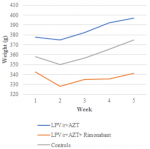
Bodyweight and adiposity: there were no differences in body weight between the treatment groups at the beginning of the study (p=0.129). All the rats gained weight during the study (Figure 1), however, after four weeks of treatment, there was a statistically significant difference in body weight between groups determined by one-way ANOVA (F(2,12) = 4.07, p = 0.045). A Tukey post hoc test revealed that rats treated with LPV/r + AZT + Rimonabant weighed significantly less (339.6 ± 22.65 g vs 398.8 ± 50.25 g) than rats treated with LPV/r + AZT only (Table 2). There was no statistically significant difference in weight between LPV/r + AZT + Rimonabant treated rats and control rats (339.6 ± 22.65 vs 371.6 ± 13.96 g).
We determined adiposity by carefully excising and weighing interscapular, inguinal, and epididymal fat depots (Table 3). Rats treated with LPV/r+AZT+Rimonabant had 58% less adiposity than rats treated with LPV/r+AZT-only (5.58 ± 1.83 vs 9.59 ± 3.72 g). They also had 32% less adiposity than control rats, (5.58 ± 1.83 vs 8.21 ± 1.32 g). These differences were not statistically significant by one-way ANOVA (p=0.073).
In this experimental study, co-administration of the cannabinoid type 1 receptor blocker, Rimonabant, with antiretroviral drugs known to produce insulin resistance (LPV/r+AZT), resulted in significantly higher insulin sensitivity in Sprague Dawley rats as determined by calculation of area under curve of graphs of blood glucose concentration plotted against time following a two-hour insulin tolerance test. The rats also had lower body weight. Besides, CBR1 antagonism resulted in smaller fat depots and leaner body composition.
The study design employed was simple and easy to replicate, while the in-vivo experiments mimic routine drug administration in the real world. The main limitation of the study is the use of the insulin tolerance test (ITT) in determining insulin sensitivity rather than the gold standard hyperinsulinemic-euglycemic clamp (HEC). Besides, the clinical use of first-generation CB1R antagonists such as Rimonabant has been suspended due to adverse central effects [18]. However, promising second-generation CBR1 antagonists with little blood-brain barrier penetration are in development [19].
In previous studies, CB1R antagonism has proven benefits on insulin sensitivity [20], however, to our knowledge, this is the first study to look into the effects of CB1R antagonism in the context of antiretroviral drug administration. Similar to the evolution of type II diabetes mellitus in the general population, both PIs and NRTIs induce insulin resistance by actions at multiple tissue targets in a multifactorial and polygenic manner [11]. In adipocytes, PIs inhibit GLUT 4 in a non-competitive dose-dependent manner while in pancreatic islets, they inhibit insulin secretion [21]. NRTI treatment, on the other hand, reduces whole-body glucose disposal [12]. Overactivity of the endogenous cannabinoid system (ECS) occurs in hyperglycaemic and obese states [5]. The mechanisms by which PIs and NRTIs induce insulin resistance appear to overlap significantly with those mechanisms activated by an overactive ECS [22]. In the present study, we hypothesised that endogenous cannabinoid activity is involved in PI and NRTI induced insulin resistance, and we demonstrated that blockade of the ECS ameliorates the insulin resistance produced by chronic treatment with the PIs Lopinavir/ritonavir alongside the NRTI, Zidovudine. The implication of these findings is that cART drugs may work cooperatively with or via the ECS to produce deleterious metabolic and physical changes.
Future studies can measure by direct assay, changes in endogenous cannabinoid activity directly either as a result of treatment with antiretroviral drugs in similar study designs or in HIV patients who present metabolic derangements while on treatment with cART. Besides, cannabinoid agonism via Dronabinol, or Δ-9-tetrahydrocannabinol, a synthetic mimic of Cannabis Sativa L., has been used to treat HIV induced anorexia successfully [23]. This observation presents an opportunity to study the nexus between caloric intake and endogenous cannabinoid tone in HIV infected patients with or without metabolic derangements.
In conclusion the present study indicates that endogenous cannabinoid tone is probably involved in the etiology of insulin resistance and disordered adiposity that accompanies use of Lopinavir/Ritonavir and AZT containing cART regimens. Enhanced endogenous cannabinoid tone is a pathophysiologic mechanism shared with metabolic syndromes of other etiologies. The study, therefore, also points to a potentially viable new approach for investigating root causes and managing insulin resistance associated with cART which still remains unsatisfactory and is a leading cause of morbidity and mortality in HIV patients.
What is known about this topic
- Overactivity of the endogenous cannabinoid system is a crucial orchestrator of metabolic disease and obesity;
- CBR 1 receptor antagonism ameliorates many of these metabolic derangements and physical changes resulting in better insulin sensitivity, lower body weight and leaner body composition in both human and animal studies.
What this study adds
- Insulin sensitivity is improved by treatment with the CBR 1 receptor antagonist, rimonabant in the context of treatment with antiretroviral drugs that are known to induce insulin resistance such as lopinavir/ritonavir and zidovudine;
- Administering the CBR 1 receptor antagonist rimonabant, along with antiretroviral drugs known to induce lipodystrophy results in a leaner body composition; these findings suggest a role for the endogenous cannabinoid system in the evolution of antiretroviral therapy induced metabolic syndrome;
- Future study designs can measure directly, endogenous cannabinoid activity in response to administration of cART in animal models and in HIV patients on cART who present with metabolic derangements and lipodystrophy.
The authors declare no competing interests.
Brian Lishenga Makamu designed and conducted the experiments as well as prepared the manuscript. Peter Waweru Mwangi helped to design the experiments and proofread the manuscript. Frederick Okonji Bukachi supervised the study. All authors read and approved the final version of the manuscript.
Mr D. Wafula and Mr J. Mugweru for providing technical assistance. The late Dr F. Babubora provided the anti-retroviral drugs used in the study.
Table 1: values for area under curve for blood glucose concentration against time graphs for each rat in the different experimental groups
Table 2: mean body weights (g) at the start and the end of the study
Table 3: weights of fat depots
Figure 1: weekly weight gain graph
- Blümer RM, van Vonderen MG, Sutinen J, Hassink E, Ackermans M, van Agtmael M a et al. Zidovudine/lamivudine contributes to insulin resistance within 3 months of starting combination antiretroviral therapy. AIDS. 2008;22(2):227-36. PubMed | Google Scholar
- Yan Q, Hruz PW. Direct comparison of the acute in vivo effects of HIV protease inhibitors on peripheral glucose disposal. J Acquir Immune Defic Syndr. 2005;40(4):398-403. PubMed | Google Scholar
- Flint OP, Noor M a, Hruz PW, Hylemon PB, Yarasheski K, Kotler DP et al. The role of protease inhibitors in the pathogenesis of HIV-associated lipodystrophy: cellular mechanisms and clinical implications. Toxicol Pathol. 2009;37(1):65-77. PubMed | Google Scholar
- Samaras K, Wand H, Law M, Emery S, Cooper D, Carr A. Prevalence of metabolic syndrome in HIV-infected patients receiving highly active antiretroviral therapy using International Diabetes Foundation and Adult Treatment Panel III criteria: associations with insulin resistance, disturbed body fat compartmental. Diabetes care. 2007;30(1):113-9. PubMed | Google Scholar
- Gruden G, Barutta F, Kunos G, Pacher P. Role of the endocannabinoid system in diabetes and diabetic complications. Br J Pharmacol . 2016 Apr;173(7):1116-27. PubMed | Google Scholar
- Côté M, Mauriège P, Bergeron J, Alméras N, Tremblay A, Lemieux I et al. Adiponectinemia in visceral obesity: impact on glucose tolerance and plasma lipoprotein and lipid levels in men. J Clin Endocrinol Metab. 2005;90(3):1434-9. PubMed | Google Scholar
- Gelfand E V, Cannon CP. Rimonabant: a cannabinoid receptor type 1 blocker for management of multiple cardiometabolic risk factors. J Am Coll Cardiol. 2006;47(10):1919-26. PubMed | Google Scholar
- Poirier B, Bidouard J-P, Cadrouvele C, Marniquet X, Staels B, O´Connor SE et al. The anti-obesity effect of rimonabant is associated with an improved serum lipid profile. Diabetes Obes Metab. 2005;7(1):65-72. PubMed | Google Scholar
- Kosmiski L, Kuritzkes D, Lichtenstein K, Eckel R. Adipocyte-derived hormone levels in HIV lipodystrophy. Antivir Ther. 2003;8(1):9-15. PubMed | Google Scholar
- Hruz PW, Murata H, Qiu H, Mueckler M. Indinavir induces acute and reversible peripheral insulin resistance in rats. Diabetes. 2002;51(4):937-42. PubMed | Google Scholar
- Koster JC, Remedi MS, Qiu H, Nichols CG, Hruz PW. HIV protease inhibitors acutely impair glucose-stimulated insulin release. Diabetes. 2003;52(7):1695-1700. PubMed | Google Scholar
- Van Vonderen MGA, Blümer RME, Hassink EAM, Sutinen J, Ackermans MT, Van Agtmael MA et al. Insulin sensitivity in multiple pathways is differently affected during zidovudine/lamivudine-containing compared with NRTI-sparing combination antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;53(2):186-193. PubMed | Google Scholar
- Woods SC. Role of the endocannabinoid system in regulating cardiovascular and metabolic risk factors. Am J Med. 2007 Mar;120(3 Suppl 1):S19-25. PubMed | Google Scholar
- Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov. 2004 Sep;3(9):771-84. PubMed | Google Scholar
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008 Mar;22(3):659-61. PubMed | Google Scholar
- Vickers SP, Webster LJ, Wyatt A, Dourish CT, Kennett GA. Preferential effects of the cannabinoid CB1 receptor antagonist, SR 141716, on food intake and body weight gain of obese (fa/fa) compared to lean Zucker rats. Psychopharmacology (Berl). 2003;167(1):103-11. PubMed | Google Scholar
- Beguinot F, Nigro C. Measurement of Glucose Homeostasis In Vivo: Glucose and Insulin Tolerance Tests. Methods Mol Biol. 2012;933:219-28. PubMed | Google Scholar
- Onakpoya IJ, Heneghan CJ, Aronson JK. Post-marketing withdrawal of anti-obesity medicinal products because of adverse drug reactions: a systematic review. BMC Med. 2016;14(1):191. PubMed | Google Scholar
- Silvestri C, Di Marzo V. Second generation CB1 receptor blockers and other inhibitors of peripheral endocannabinoid overactivity and the rationale of their use against metabolic disorders. Expert Opin Investig Drugs. 2012;21(9):1309-1322. PubMed | Google Scholar
- Mazier W, Saucisse N, Gatta-Cherifi B, Cota D. The Endocannabinoid System: Pivotal Orchestrator of Obesity and Metabolic Disease. Trends Endocrinol Metab. 2015;26(10):524-537. PubMed | Google Scholar
- Hertel J, Struthers H, Horj CB, Hruz PW. A structural basis for the acute effects of HIV protease inhibitors on GLUT4 intrinsic activity. J Biol Chem. 2004;279(53):55147-55152. PubMed | Google Scholar
- Nagappan A, Shin J, Jung MH. Role of cannabinoid receptor type 1 in insulin resistance and its biological implications. Int J Mol Sci. 2019 Apr 29;20(9):2109. PubMed | Google Scholar
- Badowski ME, Yanful PK. Dronabinol oral solution in the management of anorexia and weight loss in AIDS and cancer. Ther Clin Risk Manag. 2018;14:643-651. PubMed | Google Scholar