Effect of maternal dietary habits and gestational weight gain on birth weight: an analytical cross-sectional study among pregnant women in the Tamale Metropolis
Abdulai Abubakari, Mubarick Nungbaso Asumah, Nihad Zimpa Abdulai
Corresponding author: Mubarick Nungbaso Asumah, Department of Global and International Health, School of Public Health, University for Development Studies, Tamale Northern Region, Tamale, Ghana 
Received: 31 Oct 2022 - Accepted: 01 Jan 2023 - Published: 11 Jan 2023
Domain: Nutrition,Obstetrics and gynecology,Maternal and child health
Keywords: Birth weight, determinants, dietary patterns, gestational, weight gain, maternal dietary habits, postnatal mothers
©Abdulai Abubakari et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Abdulai Abubakari et al. Effect of maternal dietary habits and gestational weight gain on birth weight: an analytical cross-sectional study among pregnant women in the Tamale Metropolis. Pan African Medical Journal. 2023;44:19. [doi: 10.11604/pamj.2023.44.19.38036]
Available online at: https://www.panafrican-med-journal.com//content/article/44/19/full
Research 
Effect of maternal dietary habits and gestational weight gain on birth weight: an analytical cross-sectional study among pregnant women in the Tamale Metropolis
Effect of maternal dietary habits and gestational weight gain on birth weight: an analytical cross-sectional study among pregnant women in the Tamale Metropolis
![]() Abdulai Abubakari1,
Abdulai Abubakari1, ![]() Mubarick Nungbaso Asumah1,2,&, Nihad Zimpa Abdulai3
Mubarick Nungbaso Asumah1,2,&, Nihad Zimpa Abdulai3
&Corresponding author
Introduction: dietary intake and optimal gestational weight gain are important factors leading to a positive outcome for both mothers and their infants. Women who consume inadequate diet and gain inadequate weight during pregnancy are at risk of bearing a baby with low birth weight, whereas those who gain excessive weight are at increased risk of preeclampsia, having macrosomal babies, and gestational diabetes. The study aimed to assess the effect of maternal dietary intake, gestational weight on birth weight among pregnant women in Tamale Metropolis.
Methods: the study was a health-facility-based analytical cross-sectional study that involved 316 postnatal mothers. A semi-structured questionnaire was used to collect the data. Data collected were analyzed using STATA version 12. Multiple logistic regression model was estimated to determine the predictors of birth weight. Statistical significance was set at p<0.05.
Results: the study showed 17.8%, 55.9%, and 26.4% prevalence of inadequate, adequate, and excessive gestational weight gain, respectively. Although, all respondents consume supper every day, only 40.0% consumes snacks daily, 97.5% and 98.7% consumes breakfast and lunch daily respectively. Majority of the respondents (92.4%) had acceptable minimum dietary diversity. About 11.0% and 4.0% of the babies were low birth weight and macrosomic, respectively. Furthermore, the prevalence of inadequate and adequate dietary intake was, respectively, 7.6% and 92.4%. The results showed that underweight before pregnancy (BMI<18Kg/m2) (AOR=8.3, 95% CI: 6.7-15.0) and inadequate weight gain during pregnancy (AOR=4.5, 95% CI:3.9-6.5) were significant determinant of low birthweight baby.
Conclusion: on the whole, maternal body mass index and weight gain during pregnancy were strong predictors of low birth weight. Low birth weight is a major public health concern and the causes are multifaceted in natures. Therefore, to deal with low birth weight, a more holistic and multi-sectoral approaches such as behaviour change communication and comprehensive preconception care are required.
Malnutrition in pregnancy, especially in developing countries, is at the centre of many women's health issues [1]. During pregnancy, dietary intake is of great significance as far as fetal growth and development are concerned [2]. Consuming a variety of food groups by pregnant women reduces their risk of giving birth to low birth weight babies [3]. Poor nutrition in pregnancy is related to poor birth outcomes including low birth weight babies, preterm delivery, and intrauterine growth retardation [4]. In the same way, a healthy birth outcome stems from a good nutritional status [5] therefore the diet should be diverse and balanced for pregnant women [6].
In developing nations, malnutrition in pregnant women is often the result of insufficient dietary intake, poverty, poor healthcare system, heavy workload, recurrent infections, and lack of knowledge about the appropriate food to take during pregnancy [7]. Dietary intake in pregnancy is complicated by physiological changes which lead to poor dietary intake, which has negative consequences on the developing fetus and the mother [8]. For example, iron deficiency anemia in pregnant mothers is closely linked to low birth weight in newly born infants coupled with a greater risk of premature birth [9]. Moreover, malnutrition is related with retarded growth and protein-energy malnutrition due to insufficient dietary intake, especially throughout childbirth [10].
Gaining a healthy weight appropriate for pregnancy historically is a problem for women due to insufficient nutrition [11]. However, over the past few decades, excessive weight gain has become prevalent globally, affecting all age groups, including pregnant women [12]. Obesity, a worldwide phenomenon is considered more sensitive in women than in men. This is because there are heightened dietary needs during pregnancy that are important for the mother and the baby's health [13]. A population's health status can be assessed using the number of babies born with low birth weight (<2500g) [14]. Stunting and non-communicable diseases as well as child survival are link to low birthweight, therefore, there is a need for effective public health interventions that address low weight at birth [15,16]. According to United Nations Children's Fund (UNICEF) [17], the prevalence of low birth weight (LBW) in Ghana is 14.2%. The prevalence, however, is higher in Northern Ghana than the southern part of Ghana. For example a study by Abubakari et al. [18] in Northern Ghana reported low birth weight as 26% whiles Fosu et al. [19] found a prevalence of 21%, in the Brong Ahafo. Being “too big” at birth (macrosomia) is also considered an undesirable pregnancy outcome with associated elevated risk to both mother and child [20]. However, in developing countries, macrosomia is given less consideration [21].
Birthweight more than or equal to 4.0kg is regarded as macrosomic birth [22,23]. The prevalence of macrosomia is between 5% and 20% in developed countries [24]. However, the prevalence of macrosomia in developing countries appears to be sporadic. For instance, in Lu et al. [22] study, a rise from 6.0 percent in 1994 to 7.8 percent in 2005 was recorded. A recent study on macrosomic births amongst women who were obese and overweight reported a 10.9% prevalence rate [25]. Macrosomia, which is associated with obesity and far occurs ahead of adult life [26], may lead to complex birth outcomes [23]. Obesity could lead to additional risks to mothers and newborns in resource-limited countries such as Ghana.
During pregnancy, weight gain has major health implications for both mother and infant [27]. Inadequate weight gain is related to the mother´s and fetus´ health risks [28]. Insufficient gestational weight gain could lead to low birth weight and preterm childbirth [29]. Adverse maternal and fetal effects such as large for gestational age (LGA) babies, gestational diabetes mellitus (GDM), caesarean delivery, early pregnancy loss, preeclampsia, and postpartum weight retention result from excessive weight gain during pregnancy [30]. Optimal gestational weight gain contributes to positive pregnancy outcomes such as optimal growth and development of the fetus and reduced probability of mortality during pregnancy [31]. The prevalence of inadequate and excessive gestational weight gain, birth outcome and associated risk factors vary from one setting to another.
One of the most significant causes of maternal and child morbidity and mortality, especially in developed countries, is maternal malnutrition [32]. Undernutrition among pregnant women in Ghana decreased from 9 percent in 2003 to 6 percent in 2014, whereas overnutrition increased from 26 percent in 2003 to 40 percent in 2014 [33]. Among the factors that influence the nutritional status of the developing fetus, maternal nutrition is an important one [34], because a vital inter-dependence can be found amongst the overall nutritional health of the expectant mother and that of the fetus health [35]. Therefore, the nutrition, and health of women are of decisive importance during the entire period of pregnancy.
The prevalence of excessive gestational weight gain is rising and alarming, indicating a public health concern [12]. Numerous studies in developed countries on gestational weight gain indicated that more than 40% of women are gaining weight above the Optimal Institute of Medicine (IOM) ranges [12]. It is further estimated that over 75% of African American women of reproductive age are overweight or obese and this increases their already high risk for obesity-related adverse pregnancy outcomes [36].
Optimal nutrition during pregnancy is essential for the health of the mother and the developing fetus. Dietary intake is usually predicted by culture and by the type of food available for consumption in a particular locality; hence findings in one locality may not apply to all localities in Ghana. As far as this study is concerned, a review of literature showed that there are limited studies investigating the dietary habits of pregnant women in the Tamale Metropolis and their resulting relationship with birth weight. Therefore, this study aims at investigating the maternal dietary habits, gestational weight gain and their effects on birth weight in the Tamale Metropolis. Specifically, the study will assess the dietary pattern of pregnant women, prevalence of gestational weight gain, prevalence of birth weight and associated risk factors in the Tamale Metropolis.
Study area: the research was conducted in the Tamale Metropolis. The metropolis has a gross approximate proportion of land of 646.90180sqkm [37]. Geologically, near latitudes 90 161 and 90 341 North and longitudes 00 361 and 00 571 West of the metropolitan area. The inhabitants of the metropolis are made up of 223,252. The percentage of females (50.2%) is higher compared to that of males (49.7%) [37].
Study design: analytical cross-sectional study design was used. The design aided in collecting data at a point and identification of relations concerning the different variables.
Study variables: the study considered birth weight and dietary habits as dependent variables and all other factors as independent.
Study population: the study population was postnatal mothers who are permanently residing in the Tamale Metropolis of the northern region.
Inclusion criteria: postnatal mothers who were willing to participate in the study and are permanent residents of the Tamale Metropolis and have sound minds and health were included in the study.
Exclusion criteria: excluded from the study were postnatal mothers with chronic conditions such as cancer and diabetes. This is because these factors are believed to affect an individual's nutritional status. The respondent's clinical reports in the maternal and child health booklet and medical documents were used to collect this health information.
Sample size determination: the sample size was determined based on a Snedecor and Cochran formula [38]:
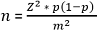
Where n=sample size , Z is the z-score of a 95% confidence level equivalent to 1.96, and p=prevalence of gestational overweight was 25.1% [18]. Where n is the sample size, Z (statistic) = 1.96, p (prevalence) = 0.29, d (margin of error) = 0.05.
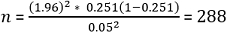
Using a 10% non- compliance and response rate, the sample size was estimated as 316. Thus, the total number of mothers to be recruited in this study is 316.
Sampling method: the study used a multistage sampling approach. The first stage used random sampling to select the health facilities in the metropolis. Consecutive sampling was used to select the study participants. On a typical data collection day, all women in attendance at the various postnatal points of the selected hospitals were approached and eligible candidates were made to sign the consent form and interviewed. This procedure was repeated on each day of data collection until the required sample size was obtained.
Data collection and instruments: the data collection was done within two months (March 2020 to May 2020). Semi structured questionnaire was used to interview respondents. The interviews were conducted in the local language (Dagbanli) for respondents who could not read and write in English or chooses to communicate in the local language. The semi-structured questionnaire was divided into sections: section A covered socio-demographic characteristics; section B covered household assets and wealth; section C covered was a food frequency questionnaire adapted from the Ghana Demographic and Health Survey report and modified to suit the purpose of the research. A check list was also developed to capture obstetric history including weigh measurements from first antenatal care (ANC) visit up to delivery and birth weight.
The socio-demographic information generated included: age, parity, marital status, educational level, and core livelihood of the respondent and the husband of married/socio-segment data essentials were: age, equality, marital status, literacy level, and center business of the respondent and the spouse whenever wedded. In assessing pregnant women's nutritional status, methods such as the use of anthropometrics was used [39]. In developing countries, the use of body mass index (BMI) in assessing nutritional status among pregnant women has been restrained since most women show up at their prenatal hospital late and so their pre-pregnancy BMI might stay unidentified [40].
Definition of variables
Ideal weight gain: the expansion of organs in lactation, growth of the fetus, and body fluid increment in volume is a result of the unceasing weight gain in pregnancy. During pregnancy, an increment of 25.0% initial body weight is considered normal. Therefore, for an average pregnant woman to reach this 25.0% initial body weight, she should gain weight of 10kg during pregnancy. An ideal weight gain is 25.0% of the initial body weight (approximately 10kg) and a lower body weight gain could be as a result of greater loss of body fat due to inadequate intake of food or a smaller gain in cell mass and extracellular fluid.
Guidelines for determining gestational weight gain: the guideline for weight gain in pregnancy was proposed by the Institute of Medicine (IOM) in the year 1990. This enabled the promotion of adequate weight gain in gestation to avert premature births. While objectives were not being entirely achieved, the Institute of Medicine guidelines for pregnancy weight gain was reorganized in 2009 to use standard body mass index (BMI) groupings which were developed by the World Health Organization (WHO) with a shift towards better maternal health outcomes [41]. The guidelines set out had three categories of gestational weight gain specifically; low or inadequate, normal or adequate, and excessive gestational weight gain. These classifications are based on the pre-pregnancy BMI.
In all antenatal care visits, the weights of expectant mothers are recorded and used as a significant clinical test in antenatal care to monitor fetal growth and development. Weight gain of mothers is also a measurement often discussed by the pregnant woman in session with her doctor or midwife. When the pregnant woman does not gain weight as the pregnancy progresses, there is a need to raise a concern. The WHO developed and instituted the recommended weight gain during pregnancy, which was based on pre-pregnancy weight. Weight gained during pregnancy was determined by subtracting the weight at first ANC from the weight at last ANC and categorized according to the IOM recommendation.
Measurement of birth weight and pre-pregnancy BMI: according to the World Health Organization, a low birth weight infant is one born with a birth weight of <2.5kg, while macrosomia is defined as birth weight ≥4.0kg [42]. Therefore, this study considered 3 categories of birth weight established by the World Health Organization which are: (1) a birth weight <2.5kg indicating low birth weight babies; (2) normal birth weight (≥2.5kg but less than 4.0 kg) and; (3) macrosomia (≥4.0kg) [42]. Pre-pregnancy BMI was determined using the weight measured during the first trimester as an appropriate proxy of pre-pregnancy weight [25,43] as weight gain during the first trimester is low (averagely 1 kg) [43]. The BMI was classified into four: (1) underweight (BMI <18.5kg/m2); (2) normal weight (18.5kg/m2 to 24.9kg/m2); (3) overweight (25kg/m2 to 29.9kg/m2) and; (4) obese (≥30kg/m2).
Measurement of individual dietary diversity score: the study aimed to assess the nutrient adequacy of the usual diet consumed during pregnancy. Respondents reported the frequency of consumption of each food on a daily, weekly and monthly basis and received 1 point if they consumed any food within the food group at least once a week during the period of pregnancy and 0 points if they rarely consumed (once a month)/never consumed the food (both in and out of home). The frequency of consumption of various food groups was analyzed by adopting the guidelines of the Food and Agricultural Organization of the United Nations for measuring household or individual dietary diversity [44]. Minimum dietary diversity for women (MDD-W) was used. Foods eaten by the respondents were classified into 10 food groups: starchy staples (cereals and white roots and tubers); dark green leafy vegetables; other vitamin A-rich fruits and vegetables; oils/fats; legumes, nuts, and seeds; other fruits and vegetables; organ meat; milk and milk products; eggs; meat and fish, seafood. The individual dietary diversity score was calculated as the sum of food groups consumed. The total individual food scores were first categorized into three, namely; low MDD-W (1-3 food groups); medium MDD-W (4-5 food groups), and high MDD-W (6 or more food groups). For further analysis, these groups were then dichotomized into two categories where 0-4 were considered low dietary diversity scores and greater than or equal to 5 food groups were considered high dietary diversity scores. The final frequency of consumption of each food group was determined on a daily and occasional basis from all food groups consumed by the women during pregnancy.
Socio-economic status: the socio-economic status was determined by summing up all household assets, totaling 14 household assets used in the questionnaire. Then, based on the number of household assets owned, an individual was classified as either low or high depending on the number of assets owned, those who owned less than 7 assets were classified as having low socioeconomic status, and those having 7 or more assets were classified as having high socioeconomic status.
Data/statistical analysis: data entry was done using Statistical Package for the Social Sciences (SPSS version 21) and transferred to STATA 12.1 for further analysis. Uniformity and plausibility checks were done after the data entry to ensure that errors were reduced. Descriptive statistics including means and standard deviations (SD) were generated for continuous variables while frequencies and percentages were generated for categorical variables. Independent t-tests were used to analyze the relationships between categorical factors and birth weight means. In cases where there were more than two groups generated, one-way ANOVA was employed to compare means. Explanatory variables under investigation were maternal age, education, occupation, parity, religion, ethnicity, husband's occupation and education, body mass index, and weight gain. The outcome variables were newborn birth weight (low birth weight, normal and macrosomia). Bivariate analyses were done using chi-square test to determine the associations between categorical variables with a statistical significance set at p<0.05. Multiple logistic regression model was estimated to determine the predictors of birth weight. All explanatory variables that were significantly associated with the outcome variable in the bivariate analyses (P<0.05) were entered into a multiple logistic regression model. P value less than 0.05 was considered statistically.
Data quality control: the first step in ensuring data quality was to provide enough training for data collectors. During the training data collectors were taught on how to administer surveys and retrieve secondary data from maternal health records books. They were also taken through how to administer a questionnaire using the appropriate interviewing skills. Because data collecting locations were widely used across the region, the completed questionnaires were collected at the end of each week for safe keeping. This guaranteed that the data obtained was verified for any necessary revisions before the next week. This also assured that the information gathered from the responders remained private from third parties. Before the data was input for analysis, all questionnaires were checked for completeness. The questionnaires were written in the English language and were translated orally into the most common locally spoken languages (mainly Dagbanli). When the questionnaire was translated, precautions were taken to ensure that the keywords or important concepts in the questionnaire were not lost. The questionnaire was originally pretested among ten women at a hospital which was not selected for the study. This was done to identify persons who would fit the requirements before the primary research study began. This was done to verify that the questionnaire conveyed the correct information and elicited the appropriate replies for the research. Before the final administration of the questionnaire, the required errors detected during the pretesting were rectified.
Ethical clearance and consent to participate: ethical clearance was obtained from the Research Committee for Human Publications and Ethics (CHRPE), Kwame Nkrumah University of Science and Technology, School of Medical Sciences, and Komfo Anokye Hospital Kumasi (CHRPE/AP/105/20) to guarantee all protocols used in the research procedure. After receiving complete information about the study, participants gave signed informed consent. However, for participants under 16 years, consent was obtained from parents or guardians. Finally, this study was conducted in accordance with the principles of the Declarations of Helsinki.
Socio-demographic characteristics: a total sample of 316 women was used in all analyses. The majority (93.7%) of the respondents reside in urban. The mean age of the respondents was 29.0 ± 5.3. The majority of respondents (90.5%) were married. Only 16.1% of the respondents had no formal education, the remaining 83.86% had some form of formal education. The most dominant religion of the respondents was Islam (75.3%). Slightly above half (52.5%) of the respondents had normal BMI. About 91% of the respondents were living in high socioeconomic status households (Table 1).
Meal pattern and food frequency of respondents: from the analysis done, 97.5% of the respondents consumed breakfast daily. Almost all respondents 98.7% consumed lunch daily while 40.0% were not consuming snacks daily. However, all respondents indicated taking supper every day. Analysis done on the frequency of food consumption indicated food was more frequently consumed in the second and third trimester compared to the first trimester (Table 2).
Dietary patterns of pregnant women: from the study, it was revealed that the more than half y (58.2%) of the women consumed starchy roots and tubers daily. Almost (97.5%) of all women consumed dark green leafy vegetables daily. Most (81.6%) of the women consumed meat and meat products daily. More than half (65.5%) of the women consumed milk and milk products occasionally, but 34.5% of the study participants consumed milk and milk products daily. Women who consumed cereal daily were more (59.8%) than those who consumed it occasionally (40.2%). Overall, majority of the respondents (92.4%) had acceptable minimum dietary diversity (Table 3).
Association between dietary diversity and maternal factors: in univariate analysis, an association between respondents' age and dietary diversity was established (p=0.010). Respondents who were widowed (100%) had adequate diet diversity while those who were cohabiting (58.3%) had inadequate dietary diversity (p=0.001). Moreover, the educational level of respondents was seen to be associated with dietary diversity (p=0.009). Furthermore, religion of the respondents was associated with dietary diversity of the respondents as 95.5%, 92.4%, and 75.0% of respondents who were Christians, Muslims, and traditionalists, respectively had diversified diets (p=0.048). Lastly, it was observed that husbands' education and occupation had an impact on dietary diversity as these were, respectively, statistically significantly associated with dietary diversity (p=0.015 and 0.001) (Table 4).
Gestational weight gain: from the study, it was revealed that slightly above half of the respondents (55.9%) gained adequate weight while (26.3%) gained excessive weight and (17.8%) gained inadequate weight.
Prevalence of low birth weight: among the respondents, the majority (85.0%) had normal birthweight at delivery while 11.0% and 4.0% respectively had low birth weight and macrosomia.
Maternal characteristics and birth weight: among the respondents, the mean birth weight was (3.1 ± 0.1kg). Babies born to mothers who were living in urban areas were on the average slightly heavier (3.1 ± 1.7kg) than those living in rural areas (2.9 ± 0.4kg). Babies of mothers within the age category of 20-30 years were averagely slightly heavier (3.1 ± 2.3kg) compared to those within the age group of 30-40 (3.1 ± 0.4kg), >40 (2.7 ± 0.1kg), and <20 (2.6 ± 0.28kg). Babies of mothers who were married were heavier (3.1 ± 1.7kg) than those who were divorced (2.9 ± 0.16kg), and cohabiting (2.5 ± 0.3kg). Newborns of mothers who had 1-3 children previously were averagely heavier (3.1 ± 1.9kg) compared to babies of mothers who were first-timers (2.8 ± 0.5kg) and those of mothers with 4-6 children (2.9 ± 0.2kg). Babies of mothers who gained optimal weight during the period of pregnancy were averagely slightly heavier (3.2 ± 2.1kg) compared to those of mothers who gained excessive weight (3.1 ± 0.3kg) and inadequate weight (2.6 ± 0.4kg) (Table 5).
Determinants of birth weight: Table 6 illustrates the relationship between the dependent and independent variables. After controlling for confounders, maternal body mass index and weight gain during pregnancy were identified as predictors of low birth weight among pregnant women. The results showed that babies of mothers who were underweight before conception (BMI <18kg/m2) were more likely to deliver a low-birth-weight child (AOR=8.3, 95%CI: 6.7-15.0, p=0.001) compared to those who had normal weight. Furthermore, pregnant women who did not gain adequate weight during pregnancy were also more likely to give birth to a low birthweight child (AOR=4.5, 95% CI: 3.9-6.5, p<0.001) compared to those who gained adequate weight.
The main aim of this study was to assess the effect of maternal dietary intake, gestational weight gain on birth weight. The study showed that mean dietary diversity score of pregnant women was 6.2 ± 0.1 SD, which is similar to finding by Ali et al. [45] who also reported a mean score (SD) of 6.17 (±0.99) and Acham et al. [46] reported a mean dietary diversity score (SD) of 6.70 (±2.22). It was established that the majority of participants in the current study had a diversified diet (more than 4 food groups), which is similar to Kenya based studies in which the majority of participants also had a diversified diet [45,47].
Furthermore, the consumption of meat and fish, as well as other fruits and vegetables, were high among study participants, contrary to other studies where the consumption of meat and meat products were low. For instance, pregnant women in Kenya were reported to consume low quantities of meat and meat products, meanwhile, in Bangladesh, 84.2% of pregnant women were reported to consume meat/fish products [48,49]. The high consumption of meat and fish among pregnant women in the Tamale Metropolis could be attributed to the location of irrigation dams near the metropolis and the rearing of livestock in and around the metropolis. Also, they take advantage of the rivers and irrigation dams to cultivate dry season gardens which provide them with sufficient vegetables like tomatoes and enough fruits. Ironically, with the availability of dry season gardens, vitamin A-rich vegetables, milk and milk products were among the least consumed among the study participants. This requires further investigation to unearth the reasons associated with low consumption of vitamin A-rich vegetables although vegetables are cultivated during dry season in the study area. These findings do not differ from Ochieng et al. [50] study in Tanzania where the majority of pregnant women did not consume milk and milk products.
In comparison with the national low birth weight estimated prevalence (13%), the estimated prevalence of low birth weight in the present study was lower. It is noteworthy to mention also that the prevalence ascertained in this study falls slightly outside of the WHO's target of less than 10% [51] but similar to the Multiple Indicator Cluster Surveys (MICS) survey prevalence (11%) in 2011. The prevalence obtained in this study could be as a result of improvements in health-related milestones in the northern region as reported in the Ghana demographic and health survey reports [52]. Macrosomia births reported in the present study is lower than reported in other studies conducted in Ghana [18,25].
In identifying predictors of low birth weight among pregnant women, maternal pre-pregnancy BMI and weight gain during pregnancy were identified as predictors of low birth weight. The study realized that maternal pre-pregnancy BMI to a large extent determined the birth weight of babies delivered by respondents. Pregnant mothers who were underweight before pregnancy were at higher risk of delivering babies with low weight compared to those who were having a normal body mass index. The above finding was in line with literature which showed that there exists an association between maternal pre-pregnancy BMI and the birth weight of new-borns [53,54]. Similarly, a study found in Beijing, China, reported that pregnant women with inadequate weight before pregnancy were at higher risk of having a low birthweight baby [55]. Available literature attributes the association between low birth weight and a BMI of less than 18.5kg/m2 to negative energy balance. In the same vein, LBW was higher in infants whose mothers were underweight during pregnancy compared to those whose mothers were normal. A systematic analysis of 42 studies showed that offspring born to underweight mothers were at greater risk of having LBW compared to those born to women with an average weight in both developed and Low Middle-Income Countries (LMICs) [56]. This finding is additionally supported by a recent meta-analysis within the context of LMICs, revealed that LBW was significantly associated with maternal underweight, but not maternal overweight/obesity [57]. These findings, therefore, require strong policy attention to address the problem of undernutrition among mothers, especially for the rationale of its intergenerational effects. This in effect presents adverse consequences on birth weight and infant and maternal mortality [58]. As undernutrition in itself a multifactorial problem, the solution would require developing cross-cutting policies and placing the issue on a broad national health and development agenda.
Lastly, the study also found an association between pregnancy weight gain and the birth weight of the child. Mothers who gained inadequate weight were more likely to deliver low-weight babies compared to their counterparts who gained adequate weight. This finding is consistent with other studies conducted in many parts of the world. For instance, a study conducted by Lima et al. [59] in Brazil found that the effect of gestational weight gain on the increase in birth weight was greater than that of pre-pregnancy body mass index (P-value of 0.001). Similar findings have been reported by studies conducted in Beijing, China, that women with inadequate gestational weight gain had a higher risk of low birth weight and small for gestational age infants [55]. Moreover, several studies have shown that the birth weight of the baby was significantly associated with gestational weight gain [60,61].
As with cross sectional studies, the cause-effect relationship is not intended. Besides cross-sectional studies have inherent recall bias. However, this was unlikely to alter the results of the present study as effort was made assist respondents to provide appropriate responses through probing. Also, the study was conducted among women who attended ANC and delivered in a health facility. Since some women still give birth in their homes in Northern Ghana, the generalisation of the findings emanating from this study should be done with caution. As with retrospective study, the study was prone to recall bias. To mitigate this, the study participants selected mothers who were attending postnatal care services at selected health facilities in the metropolis. With this most of the data were recorded already in the maternal and child health record book. Moreover, effort was made to assist respondents to provide appropriate responses through probing, hence recall bias was unlikely to alter the results of the present study.
The study showed that a double burden of malnutrition (low birth weight and macrosomia) coexisted among new-borns in the Tamale Metropolis, which is currently being experienced by developing and transition counties. Pre-pregnancy BMI and weight gain during pregnancy were found to predict birth weight. Low birth weight is a major public health concern and the causes multifaced in natures. Therefore, in order to deal with it a more holistic and multi-sectoral approaches such as behaviour change communication and comprehensive preconception care are required.
What is known about this topic
- Dietary consumption during pregnancy has a significant impact on the growth and development of the fetus;
- Pregnancy-related physiological changes make it difficult to maintain a healthy diet, which has serious detrimental effects on both the mother and the growing fetus;
- Adverse maternal and fetal effects such as large gestational age (LGA) babies, gestational diabetes mellitus (GDM), caesarean delivery, early pregnancy loss, preeclampsia, and postpartum weight retention result from excessive weight gain.
What this study adds
- The double burden of malnutrition coexisted among infants born in the Tamale Metropolis comprising of low birth weight (undernutrition) and macrosomia (overnutrition), which is currently being experienced by developing and transition counties;
- This research also reveals valuable details on the birth weight determinants of babies born in the Tamale Metropolis;
- Maternal body mass index and weight gain during pregnancy were strong predictors of low birth weight among pregnant women.
The authors declare no competing interests.
Conceptualization: Nihad Zimpa Abdulai, Abdulai Abubakari; data collection: Nihad Zimpa Abdulai; data curative: Nihad Zimpa Abdulai, Abdulai Abubakari, Mubarick Nungbaso Asumah; formal analysis: Nihad Zimpa Abdulai, Abdulai Abubakari, Mubarick Nungbaso Asumah; project supervision: Abdulai Abubakari; original draft: Mubarick Nungbaso Asumah; review and editing: Nihad Zimpa Abdulai, Abdulai Abubakari, Mubarick Nungbaso Asumah. All the authors proof read and approved the final version of this manuscript.
We are grateful to Tamale Metropolitan Director of Health Services and his team for the support and permission granted to us in order to undertake our studies in the district. In addition, we are indebted to the participants who voluntarily decided to share their experiences to enrich this research.
Table 1: sociodemographic characteristics of respondents
Table 2: meal pattern and food frequency of respondents
Table 3: dietary patterns of pregnant women
Table 4: association between dietary diversity and maternal factors
Table 5: maternal characteristics/factors and birth weight
Table 6: determinants of birthweight
- Victora CG, Christian P, Vidaletti LP, Gatica-Domínguez G, Menon P, Black RE. Revisiting maternal and child undernutrition in low-income and middle-income countries: variable progress towards an unfinished agenda. Lancet. 2021;397(10282):1388-99. PubMed | Google Scholar
- Dörsam AF, Preißl H, Micali N, Lörcher SB, Zipfel S, Giel KE. The Impact of Maternal Eating Disorders on Dietary Intake and Eating Patterns during Pregnancy: A Systematic Review. Nutrients. 2019;11(4):840. PubMed | Google Scholar
- Abubakari A, Jahn A. Maternal Dietary Patterns and Practices and Birth Weight in Northern Ghana. PLoS One. 2016;11(9):e0162285. PubMed | Google Scholar
- Amjad S, MacDonald I, Chambers T, Osornio-Vargas A, Chandra S, Voaklander D et al. Social determinants of health and adverse maternal and birth outcomes in adolescent pregnancies: A systematic review and meta-analysis. Paediatr Perinat Epidemiol. 2019 Jan;33(1):88-99. PubMed | Google Scholar
- Wu G, Bazer FW, Cudd TA, Meininger CJ, Spencer TE. Maternal nutrition and fetal development. J Nutr. 2004 Sep;134(9):2169-72. PubMed | Google Scholar
- Black RE, Allen LH, Bhutta ZA, Caulfi LE, Onis M De, Ezzati M et al. Maternal and Child Undernutrition 1 Maternal and child undernutrition: global and regional. Lancet. 2008;243-60.
- Ahmed T, Hossain M, Sanin KI. Global burden of maternal and child undernutrition and micronutrient deficiencies. Ann Nutr Metab. 2012;61 Suppl 1:8-17. PubMed | Google Scholar
- Zakaria H, Laribick DB. Socio-economic determinants of dietary diversity among women of child bearing ages in Northern Ghana. UDSspace. 2014;34. Google Scholar
- Wang L, Mei Z, Li H, Zhang Y, Liu J, Serdula MK. Modifying effects of maternal Hb concentration on infant birth weight in women receiving prenatal iron-containing supplements: A randomised controlled trial. Br J Nutr. 2016 Feb;115(4):644-9. PubMed | Google Scholar
- Adikari AMNT, Sivakanesan R, Wijesinghe DGNG, Liyanage C. Assessment of nutritional status of pregnant women in a rural area in Sri Lanka. Trop Agric Res. 2016 Jul;27(2):203. Google Scholar
- Samura T, Steer J, Michelis LD, Carroll L, Holland E, Perkins R. Factors Associated With Excessive Gestational Weight Gain: Review of Current Literature. Glob Adv Health Med. 2016;5(1):87-93. PubMed | Google Scholar
- Yao R, Park BY, Foster SE, Caughey AB. The association between gestational weight gain and risk of stillbirth: a population-based cohort study. Ann Epidemiol. 2017 Oct;27(10):638-644.e1. PubMed | Google Scholar
- Papazian T, Abi Tayeh G, Sibai D, Hout H, Melki I, Rabbaa Khabbaz L. Impact of maternal body mass index and gestational weight gain on neonatal outcomes among healthy Middle-Eastern females. PLoS One. 2017 Jul;12(7):e0181255. PubMed | Google Scholar
- Manna N, Sarkar J, Baur B, Basu G, Bandyopadhyay L. Socio-Biological Determinants of Low Birth Weight: A Community based study from rural field practice area of Medical College, Kolkata, West Bengal (India). IOSR J Dent Med Sci. 2013;4(4):33-9.
- Lawn JE, Cousens S, Zupan J; Lancet Neonatal Survival Steering Team. 4 Million neonatal deaths: When? Where? Lancet. 2005 Mar 5-11;365(9462):891-900. PubMed | Google Scholar
- Lee SE, Talegawkar SA, Merialdi M, Caulfield LE. Dietary intakes of women during pregnancy in low- and middle-income countries. Public Health Nutr. 2013 Aug;16(8):1340-53. PubMed | Google Scholar
- United Nations Children's Fund (UNICEF). The state of the world´s children, 2013. New York, USA: UNICEF. 2013.
- Abubakari A, Kynast-Wolf G, Jahn A. Maternal Determinants of Birth Weight in Northern Ghana. PLoS One. 2015 Aug 17;10(8):e0135641. PubMed | Google Scholar
- Fosu MO, Abdul-Rahaman I, Yekeen R. Maternal Risk Factors for Low Birth Weight in a District Hospital in Ashanti Region of Ghana. Res Obstet Gynecol. 2013;2(4):48-54.
- Wendland EM, Torloni MR, Falavigna M, Trujillo J, Dode MA, Campos MA et al. Gestational diabetes and pregnancy outcomes - a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth. 2012 Mar;12:23. PubMed | Google Scholar
- Abubakari A, Kynast-Wolf G, Jahn A. Prevalence of abnormal birth weight and related factors in Northern region, Ghana. BMC Pregnancy Childbirth. 2015 Dec 15;15:335. PubMed | Google Scholar
- Lu Y, Zhang J, Lu X, Xi W, Li Z. Secular trends of macrosomia in southeast China, 1994-2005. BMC Public Health. 2011 Dec;11:818. PubMed | Google Scholar
- Koyanagi A, Zhang J, Dagvadorj A, Hirayama F, Shibuya K, Souza JP et al. Macrosomia in 23 developing countries: An analysis of a multicountry, facility-based, cross-sectional survey. Lancet. 2013 Feb;381(9865):476-83. PubMed | Google Scholar
- Henriksen T. The macrosomic fetus: a challenge in current obstetrics. Acta Obstet Gynecol Scand. 2008;87(2):134-45. PubMed | Google Scholar
- Addo VN. Body Mass Index, Weight Gain during Pregnancy and Obstetric Outcomes. Ghana Med J. 2010 Jun;44(2):64-9. PubMed | Google Scholar
- Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003 Apr;11(4):496-506. PubMed | Google Scholar
- Institute of medicine and National Research Council. Composition and Components of Gestational Weight Gain: Physiology and Metabolism. In: Rasmussen KM, Yaktine AL, editors. Weight gain during pregnancy: Reexamining the guidelines. The National Academies Press (US); 2009;71-110.
- Garay SM, Sumption LA, Pearson RM, John RM. Risk factors for excessive gestational weight gain in a UK population: a biopsychosocial model approach. BMC Pregnancy Childbirth. 2021;21(1):43. PubMed | Google Scholar
- Bengtson M-B, Martin CF, Aamodt G, Vatn MH, Mahadevan U. Inadequate gestational weight gain predicts adverse pregnancy outcomes in mothers with inflammatory bowel disease: results from a prospective US pregnancy cohort. Dig Dis Sci. 2017;62(8):2063-9. PubMed | Google Scholar
- Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ. 2017 Feb 8;356:j1. PubMed | Google Scholar
- Champion ML, Harper LM. Gestational Weight Gain: Update on Outcomes and Interventions. Curr Diab Rep. 2020;20(3):11. PubMed | Google Scholar
- Triunfo S, Lanzone A. Impact of maternal under nutrition on obstetric outcomes. J Endocrinol Invest. 2015;38(1):31-8. PubMed | Google Scholar
- Ghana Statistical Service (GSS), Ghana Health Service (GHS) I. Ghana maternal health survey 2017. Accra. 2018.
- Veena SR, Gale CR, Krishnaveni GV, Kehoe SH, Srinivasan K, Fall CH. Association between maternal nutritional status in pregnancy and offspring cognitive function during childhood and adolescence; a systematic review. BMC Pregnancy Childbirth. 2016 Aug 12;16:220. PubMed | Google Scholar
- Bhanbhro S, Kamal T, Diyo RW, Lipoeto NI, Soltani H. Factors affecting maternal nutrition and health: A qualitative study in a matrilineal community in Indonesia. PLoS One. 2020;15(6):e0234545. PubMed | Google Scholar
- Goodrich K, Cregger M, Wilcox S, Liu J. A qualitative study of factors affecting pregnancy weight gain in African American women. Matern Child Health J. 2013;17(3):432-40. PubMed | Google Scholar
- Ghana Statistical Service (GSS). Population and housing census: national analytical report. Accra-Ghana Ghana Stat Serv. 2010;2013.
- Snedecor GW, Cochran WG. Statistical methods, 8thEdn. Ames Iowa State Univ Press Iowa. 1989;54:71-82.
- Gibson RS. Principles of nutritional assessment. Oxford University Press, USA. 2005. Google Scholar
- Kruger HS. Maternal anthropometry and pregnancy outcomes: a proposal for the monitoring of pregnancy weight gain in outpatient clinics in South Africa. Curationis. 2005;28(4):40-9. PubMed | Google Scholar
- Restall A, Taylor RS, Thompson J, Flower D, Dekker GA, Kenny LC et al. Risk factors for excessive gestational weight gain in a healthy, nulliparous cohort. J Obes. 2014;2014:148391. PubMed | Google Scholar
- World Health Organization. UNICEF-WHO low birthweight estimates: levels and trends 2000-2015. World Health Organization. 2019. Google Scholar
- Shin D, Chung H, Weatherspoon L, Song WO. Validity of prepregnancy weight status estimated from self-reported height and weight. Matern Child Health J. 2014;18(7):1667-74. PubMed | Google Scholar
- Food and Agriculture Organization of the United Nations (FAO). Guidelines for measuring household and individual dietary diversity. 2013.
- Ali F, Thaver I, Khan SA. Assessment of dietary diversity and nutritional status of pregnant women in Islamabad, Pakistan. J Ayub Med Coll Abbottabad. 2014;26(4):506-9. PubMed | Google Scholar
- Hedwig A, Wilna HO, Abdulkadir AE. Dietary diversity, micronutrient intake and their variation among black women in informal settlements in South Africa: A cross-sectional study. Int J Nutr Metab. 2012;4(2):24-39. Google Scholar
- Kiboi W, Kimiywe J, Chege P. Determinants of dietary diversity among pregnant women in Laikipia County, Kenya: a cross-sectional study. BMC Nutr. 2017;3(1):1-8. Google Scholar
- Kiboi MN, Ngetich KF, Diels J, Mucheru-Muna M, Mugwe J, Mugendi DN. Minimum tillage, tied ridging and mulching for better maize yield and yield stability in the Central Highlands of Kenya. Soil Tillage Res. 2017;170:157-66. Google Scholar
- Nguyen PH, Sanghvi T, Kim SS, Tran LM, Afsana K, Mahmud Z et al. Factors influencing maternal nutrition practices in a large scale maternal, newborn and child health program in Bangladesh. PLoS One. 2017;12(7):e0179873. PubMed | Google Scholar
- Ochieng J, Afari-Sefa V, Lukumay PJ, Dubois T. Determinants of dietary diversity and the potential role of men in improving household nutrition in Tanzania. PLoS One. 2017;12(12):e0189022. PubMed | Google Scholar
- World Health Organization (WHO). Resolution WHA 65.6. Comprehensive implementation plan on maternal, infant and young child nutrition. Sixty-fifth World Health Assembly, Geneva, 21-26 May 2012. Resolutions and decisions, annexes. World Health Organization Geneva (Switzerland); 2012.
- Ghana Statistical Service (GSS). Ghana Demographic and Health Survey 2014: Ghana Statistical Service, Ghana Health Service. Ghana Statistical Service (GSS) Ghana Demographic and Health Survey. 2014.
- Lecorguillé M, Jacota M, de Lauzon-Guillain B, Forhan A, Cheminat M, Charles M-A et al. An association between maternal weight change in the year before pregnancy and infant birth weight: ELFE, a French national birth cohort study. PLoS Med. 2019;16(8):e1002871. PubMed | Google Scholar
- McCloskey K, Ponsonby A, Collier F, Allen K, Tang MLK, Carlin JB et al. The association between higher maternal pre-pregnancy body mass index and increased birth weight, adiposity and inflammation in the newborn. Pediatr Obes. 2018;13(1):46-53. PubMed | Google Scholar
- Zhang CH, Liu XY, Zhan YW, Zhang L, Huang YJ, Zhou H. Effects of Prepregnancy Body Mass Index and Gestational Weight Gain on Pregnancy Outcomes. Asia Pac J Public Health. 2015;27(6):620-30. PubMed | Google Scholar
- Dharmalingam A, Navaneetham K, Krishnakumar CS. Nutritional status of mothers and low birth weight in India. Matern Child Health J. 2010;14(2):290-8. PubMed | Google Scholar
- Rahman MM, Abe SK, Kanda M, Narita S, Rahman MS, Bilano V et al. Maternal body mass index and risk of birth and maternal health outcomes in low-and middle-income countries: a systematic review and meta-analysis. Obes Rev. 2015;16(9):758-70. PubMed | Google Scholar
- Amugsi DA, Lartey A, Kimani-Murage E, Mberu BU. Women´s participation in household decision-making and higher dietary diversity: findings from nationally representative data from Ghana. J Heal Popul Nutr. 2016;35(1):16. PubMed | Google Scholar
- Lima RJCP, Batista RFL, Ribeiro MRC, Ribeiro CCC, Simões VMF, Lima Neto PM et al. Prepregnancy body mass index, gestational weight gain, and birth weight in the BRISA cohort. Rev Saude Publica. 2018;52:46. PubMed | Google Scholar
- AlShammari E, Bano R, Banu S, Fatima SB, Sattam A. Study on the determinants of pregnancy outcome. 2015. Google Scholar
- Pal R, Maiti M, Roychoudhury B, Sanyal P, Chowdhury B. Association of Pregestational BMI and Antenatal Weight Gain With Pregnancy Outcome: A Prospective Observational Cohort Study. Int J Womens Heal Reprod Sci. 2017;5(1):37-40. Google Scholar











