Distinct pattern and prevalence of Plasmodium falciparumdihydropteroate synthase gene mutations in children with sickle cell anaemia and haemoglobin AA in Benin City, Nigeria: the impact of HbAA
Izehiuwa Gertrude Enato, Ayebo Evawere Sadoh, Okoeguale Michael Ibadin, Magdalene Erhieyouvbe Odunvbun, Iriagbonse Iyabo Osaigbovo
Corresponding author: Izehiuwa Gertrude Enato, Institute of Child Health, University of Benin, Benin City, Edo State, Nigeria| Edo State University, Uzairue, Edo State, Nigeria 
Received: 18 Mar 2022 - Accepted: 02 Jul 2022 - Published: 13 Oct 2022
Domain: Pediatric hematology,Pediatrics (general)
Keywords: Molecular markers, sulphadoxine resistance, Plasmodium falciparum dihydropteroate synthase
©Izehiuwa Gertrude Enato et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Izehiuwa Gertrude Enato et al. Distinct pattern and prevalence of Plasmodium falciparumdihydropteroate synthase gene mutations in children with sickle cell anaemia and haemoglobin AA in Benin City, Nigeria: the impact of HbAA. Pan African Medical Journal. 2022;43:80. [doi: 10.11604/pamj.2022.43.80.34334]
Available online at: https://www.panafrican-med-journal.com//content/article/43/80/full
Research 
Distinct pattern and prevalence of Plasmodium falciparumdihydropteroate synthase gene mutations in children with sickle cell anaemia and haemoglobin AA in Benin City, Nigeria: the impact of HbAA
Distinct pattern and prevalence of Plasmodium falciparum dihydropteroate synthase gene mutations in children with sickle cell anaemia and haemoglobin AA in Benin City, Nigeria: the impact of HbAA
![]() Izehiuwa Gertrude Enato1,2,&,
Izehiuwa Gertrude Enato1,2,&, ![]() Ayebo Evawere Sadoh2,3, Okoeguale Michael Ibadin3, Magdalene Erhieyouvbe Odunvbun3,
Ayebo Evawere Sadoh2,3, Okoeguale Michael Ibadin3, Magdalene Erhieyouvbe Odunvbun3, ![]() Iriagbonse Iyabo Osaigbovo4,5
Iriagbonse Iyabo Osaigbovo4,5
&Corresponding author
Introduction: specific mutations on the Plasmodium falciparum dihydropteroate synthase (Pfdhps) gene mediate sulphadoxine/pyrimethamine (SP) resistance and thus, pose a threat to the efficacy of SP-Intermittent Preventive Therapy (SP-IPT) in malaria chemoprevention in children, including those with sickle cell anaemia (SCA). This study determined the distinct pattern and prevalence of Pfdhps mutations in children with SCA and in those with homozygous haemoglobin A (HbAA) in Benin City, Nigeria; showing the impact of haemoglobin phenotype.
Methods: this was a cross-sectional study involving children with SCA and HbAA. Those with successfully amplified Pfdhps genes were included in the study. Point mutations and mutant haplotypes of the Pfdhps gene were identified. Parasite density (PD) was determined by estimating the parasite numbers/μl of blood from the thick film. Descriptive, univariable and multivariable analysis were used appropriately.
Results: a total of 146 children: 71 with SCA and 75 with HbAA were recruited, with a mean age of 46.6 ± 13.0 and 36.4 ± 17.6 respectively; proportion of males were 45(63.4%) and 43(57.3%) respectively. I431V, S436A, A437G, A581G, and A613G mutations were present; but the K540E mutation was absent. ISGKAA 41(28.1%) and VAGKGS 61(41.8%) were the most prevalent mutant haplotypes in this study. The prevalence of VAGKGS haplotype 43(57.3%) was significantly higher in HbAA group compared to that 18(25.4%) in the SCA group (p < 0.001). The prevalence of ISGKAA in SCA group 25(35.2%) was significantly higher than that 16(21.3%) in the HbAA group (p=0.032). HbAA phenotype was the only significant predictor for the presence of the VAGKGS mutant haplotype (aOR:3.0, 95%CI: 1.375 to 6.499; p=0.006).
Conclusion: the HbAA phenotype was a significant predictor for the occurrence of the quintuple mutant haplotype (VAGKGS). The K540E mutation was absent; thus, SP-IPT can be explored in children younger than five years with SCA.
In children with sickle cell anaemia (SCA), Sulphadoxine/Pyrimethamine as Intermittent Preventive Treatment (SP-IPT), has been shown to be efficacious and effective in reducing malaria induced morbidity and mortality [1,2]. However, point mutations on Plasmodium falciparum dihydrofolate reductase (pfdhfr) and Plasmodium falciparum dihydropteroate synthase (pfdhps) genes pose a threat to the efficacy of SP-IPT; these mutations form the molecular markers for sulphadoxine/pyrimethamine (SP) resistance [3]. Molecular markers for pyrimethamine resistance were established long before SP resistance [4]; the pfdhfr mutations have become fixed in the general population [5,6]. SP resistance was heralded by point mutations on the P. falciparum dihydropteroate synthase (pfdhps) gene at codons 436 (S436A), 437 (A437G), 540 (K540E), 581 (A581G), and 613 (A613S/T) [7-10].
SP-IPT (in infants and young children) is said to remain efficacious in areas where the prevalence of pfdhps K540E is less than 50%, even in the presence of significant SP resistance and malaria treatment failure, which is indicated by the quintuple mutant haplotype: 3DHFR (N51I+C59R+S108N) + 2DHPS (A437G+K540E) [11-13]. A high prevalence (50% or more) of pfdhps K540E mutant allele in a region, is indicative of failure of SP as IPT in those with little or no malaria immunity [11-13]. Thus, SP-IPT can be recommended in regions with low prevalence (< 50%) of pfdhps K540E mutations [11]. A few studies have described an emerging pfdhps I431V mutation and pfdhps quintuple mutant haplotype VAGKGS (I431V, S436A, A437G, K540 A581G and A613S) [6,14,15].
White et al. and Pongtavornpinyo et al. mathematical models, further described that mutations such as pfdhps mutations on malaria parasites are statistically more likely to occur and spread from infections with a large parasite biomass [16,17]. Infants and young children with normal haemoglobin phenotype (HbAA) have a higher parasite biomass/density compared to their counterparts with homozygous haemoglobin S (HbSS) as seen in those with SCA, with a lower parasite biomass [18,19]. Therefore, the occurrence and spread of mutant genes (thus SP resistance) may be higher in infants and young children with HbAA compared to those with sickle cell anaemia. The aim of this study was to determine and compare the prevalence and distinct pattern of pfdhps mutations in children aged 6-59 months with SCA and those with HbAA in Benin City, Nigeria; showing the impact of haemoglobin phenotype (HbAA and HbSS:SCA) and parasite density.
Study design and setting
This was a cross-sectional comparative study, which was carried out at the University of Benin Teaching Hospital (UBTH), Sickle Cell Centre (SCC) and Children´ Clinic at The Central Hospital all in Benin City, Edo State, Nigeria. Edo State is located in the South-south geographical region of Nigeria, where malaria transmission is holoendemic and stable. UBTH, Benin City provides primary, secondary and tertiary health care services to the people living in the immediate five Local Government Areas of Egor, Ikpoba-Okha, Oredo, Ovia North-East and Ovia South-West of Edo State. Sickle Cell Centre (SCC), is attached to the Central Hospital, Benin City. SCC provides secondary health care services to population within and outside Benin City. The SCC manages only sickle cell disease (SCD) patients (children and adults); clinics are run daily.
Sample population
Children with SCA aged 6-59 months were recruited from the sickle cell clinic in UBTH and the Sickle Cell Centre attached to the Central Hospital, Benin City; while children with HbAA aged 6-59 months were recruited from The General Practice Clinic of UBTH, and Children´ clinic of the Central Hospital, Benin. Minimum sample size was calculated using the formula for comparing two proportions (HbAA and SCA groups) [20], using 95% confidence interval (CI). The minimum sample size was 164 for each study group.
Inclusion criteria: children aged 6-59 months with SCA with Haemoglobin SS phenotype or haemoglobin AA phenotype (confirmed by electrophoresis using cellulose acetate paper); and with P. falciparum parasite seen on blood smear determined using microscopy.
Exclusion criteria: all children on sulpha-based medication/chemoprophylaxis, such as trimethoprim/sulphametoxazole. This information was obtained from the care givers/parents. Study participants were recruited following screening of all children aged 6-59 months visiting the respective clinics. Patients who met the inclusion criteria were recruited consecutively until sample size of 164 for each study group was met.
Data collection: at screening and recruitment, a proforma was used to obtain information from the parents or guardians on the child´s age, date of birth, and sex; information on drug history, including use of sulpha-based medication such as co-trimoxazole - trimethoprim/sulphamethoxazole. Three millilitres of blood was aseptically obtained from peripheral veins of the participants for Haemoglobin electrophoresis (to determine/confirm the Haemoglobin genotype of study participants); thick and thin blood film for malaria parasite and parasite density. Blood was collected on filter paper for dried blood spot (DBS). At the end of sample collection, the filter paper dried blood spots positive for P. falciparum following screening by microscopy, were selected and transported in a desiccant via courier to the London School of Tropical Medicine and Hygiene (LSTMH), United Kingdom. Parents/guardians were counseled following results of microscopy and haemoglobin genotype for malaria parasite. All patients with malaria parasitaemia received prescription for Artemisinin based Combination Therapy (ACT), irrespective of the absence/presence of symptoms.
Laboratory analysis
Determination of Haemaglobin phenotype
This was done using cellulose acetate method at pH 8.4. This took place in the Medical Haematology Laboratory, University of Benin Teaching Hospital and the Haematology Laboratory of the Central Hospital, Benin. One milliliter of blood collected from each child was transferred into a test tube. The red blood cells were washed in 0.9% normal saline by adding 9mls of normal saline to 1 ml of blood in the test tube. The solution was then centrifuged at 5000rpm for 5 minutes. The supernatant was discarded, leaving the cells. Three milliliters of water was then added to 1ml of the cells to cause lysis of the red cells. With the use of a pipette (GIBSON, made in France) and a disposable plastic pipette, 0.1ml of the resultant solution (haemolysate) was applied to the cellulose acetate paper and the paper then placed in the electrophoresis chamber for 30 minutes. The rate of migration on the electrophoretic machine (Beckman electrophoresis machine Model R-120, made in Germany) was used to determine the haemoglobin genotype.
Thick and thin blood film for malaria parasite and speciation using Geimsa stain: i) Each clean grease-free slide was labelled with the date and the sample number, age and sex of the patient. ii) Using a pipette (GIBSON, made in France) and a disposable plastic pipette tip, a drop of blood from the subject was made at one end of the labelled slide. iii) A smear of blood was then made (using the plastic cap of the needle) with the drop of blood, covering an area of 15X15 mm. iv) On the same slide, a drop of blood was applied 10 mm away from the thick smear. v) The blood smear was spread using the smooth edge of a slide spreader to make a thin film of blood. To make the thin film of blood, the slide spreader was placed at 45 degrees to the horizontal just behind the blood spot. This was to allow the blood spread by capillary action against the edge of the slide spreader. Then with a single smooth swipe, the spreader was moved towards the edge opposite the thick film so that a thin film with a “tail” is formed. vi) The slide containing the thick and thin blood was allowed to air-dry. vii) After thorough drying, the slide was placed horizontally on a staining rack. Viii) A small drop of methanol was then applied to the thin film. The thin film was then allowed to fix for one minute. ix) After fixing the thin blood smear, the slides were placed facing downwards in a staining trough. x) The Geimsa stain was then slowly poured into the staining trough and allowed to stand for 30 minutes using 3% Geimsa solution (freshly prepared). xi) After 30 minutes, the stain was flushed off the slides with clean tap water. xii) The slide was then allowed to air dry. xiii) When the thick film was completely dry, a drop of immersion oil was then applied on the film. xiv) The oil was spread to cover an area about 10 millimeters in diameter. xv) Using 100X objective magnification, the smear was examined for malaria parasites and pigments. The result was then reported as positive if malaria parasite was seen. If no malaria parasite seen, it was reported as NPF (No parasites found). xvi) The different plasmodium species were identified on the thin film using 100X objective to examine for the parasites.
DNA Extraction, nested PCR amplification and direct sequencing
The analysis was carried out in stages; DNA extraction from bloodspots on ?lter paper was carried out first using the Chelex method in a 96-well plate format as described by Plowe et al. in 1995 [21]. DNA extraction was followed by nested PCR amplification of the pfdhps genes. 711 bp products of pfdhps genes were sized against 100 bp molecular weight marker on 1.2% agarose gel stained with ethidium bromide. Exonuclease I-Fast Alkaline Phosphatase was used to enzymatically purify PCR products, according to the manufacturer´s instructions, followed by direct sequencing of products. Point mutations at codons 431, 436, 437, 540, 581 and 613 of the pfdhps gene were read and recorded using Chromas software 2.4.
Determination of parasite density (degree of parasitaemia): counting parasite numbers was done by estimating parasite numbers/Ál of blood from the thick film. i) The average number of parasites per HPF was counted on the thick film. Twenty fields were examined to determine the average number of parasites per HPF. ii) The number of parasites counted per HPF was multiplied by a factor of 500. The computed value was parasite numbers/Ál of blood (parasitaemia) for the individual. Parasite density =250 000 parasites/Ál of blood is one of the laboratory criteria for severe malaria.
Definition of variables
Dependent variables: Pfdhps point mutations: Plasmodium falciparum dihydropteroate synthase point mutations at codons 431, 436, 437, 540, 581 and 613 (I431V, S436A, A437G, K540E, A581G and A613S respectively). Pfdhps mutant haplotypes: Plasmodium falciparum dihydropteroate synthase haplotypes; a combination of point mutations (single, double, triple, quadruple and quintuple mutant haplotypes).
Independent variables: SCA/HbSS: sickle cell anaemia/ Homozygous Haemoglobin SS; HbAA: Homozygous Haemoglobin AA; GMPD: Geometric Mean Parasite Density - parasite density; Age: 6-59 months; Gender: Male and Female.
Statistical analysis
All data generated were collated, checked and analysed using statistical package for social sciences (SPSS) version 17.0 (SPSS for Window Inc; Chicago, LL, USA). Gender distribution and prevalence of Pfdhps point mutations and mutant haplotypes were recorded as simple percentages. Continuous variables (age and parasite density) were recorded as means and standard deviation. Logarithmic transformation was applied to all values recorded for parasite density to make the data normally distributed and amenable to statistical analysis. Parasite density was recorded as geometric mean parasite density (GMPD). Test of association between variables, such as prevalence of Pfdhps point mutations/mutant haplotypes in children with SCA and HbAA was done using Chi-square. The GMPD between two groups: (those with the presence and those with absence of Pfdhps point mutations/mutant haplotypes) was compared using independent T-test. Mean difference across more than two categories of variables (GMPD across mutant Pfdhps haplotypes) was done using one-way Analysis of Variance (ANOVA). Multiple logistic regressions model was used to identify predictors (Haemogblobin phenotype and Parasite density) of molecular markers of resistance. Statistical significance was set at p < 0.05.
Ethical consideration: ethical approval for the study was obtained from the Ethics and Research Committee of University of Benin Teaching Hospital (UBTH), Benin City; UBTH (ADM/E22/A/VOL.VII/839). A written informed consent was obtained from parents and/or guardians of the study participants by the investigators before samples were collected.
A total of 164 children with SCA and 164 children with HbAA, who had Plasmodium falciparum parasites/isolates demonstrated using microscopy, were recruited for this study. However, following DNA Analysis of dried blood spots, only 71 (43.3%) children with SCA and 75 (45.7%) children with HbAA had successfully amplified P. falciparum dhps genes.
Age and sex distribution of study participants
Of the 71 children with SCA and 75 children with HbAA and amplified P.falciparum dhps gene, males were 45(63.4%) and 43(57.3%) respectively (Table 1). The mean age ±SD of children with amplified Pfdhps in the SCA group 46.6±13.0 was statistically significantly higher than that 36.4±17.6 observed in the HbAA group (t=3.92, p < 0.001). The characteristics of both study groups are shown in Table 1.
Pattern and prevalence of mutant pfdhps point mutations and pfdhps haplotypes
The pfdhps point mutations found, and their prevalence were I431V 82/146 (56.2%), S436A 94/146 (64.4%), A437G 144/146 (98.6%), A581G 83/146 (56.8%) and A613S 83/146 (56.8%) (Table 2). The K540E mutation was totally absent in this study. The different mutant pfdhps haplotypes identified in the study were single mutant haplotype: ISGKAA 41(28.1%) and IAAKAA 2(1.4%); the double mutant haplotype: IAGKAA 5(3.4%) and ISGKGA 4(2.75); triple mutant haplotype: IAGKAS 7(4.8%), IAGKGA 1(0.7%), ISGKGS 1(0.7), VAGKAA 1(0.7%), and VSGKGA 1(0.7%); quadruple mutant haplotype: IAGKGS 2(1.4%), VAGKGA 5(3.4%), VAGKAS 3(2.1%); and the quintuple mutant haplotype: VAGKGS 61(41.8%), (Table 2). Each of these pfdhps mutant haplotypes was found in both groups (SCA and HbAA), except the IAAKAA, VAGKAS, VSGKGA, VAGKAA haplotypes which were found only in SCA group and the ISGKGA found only in HbAA group (Annex 1).
Pattern and prevalence of pfdhps point mutations and mutant haplotypes in children with SCA and in those with HbAA
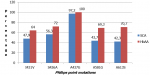
The prevalence of S436A: 54(72.0%), A581G: 52(69.3%) and A613S: 53(70.7%) mutations in HbAA group were statistically significantly higher than 40(56.3%), 31(43.7%) and 30(42.3%) respectively found in the SCA group (p=0.048 for S436A, p=0.002 for A581G; and p=0.001 for A613S) (Figure 1). The point mutation with the highest prevalence in the study population was the A437G mutation; with a prevalence of 69(97.2%) in SCA group, which was lower than 75(100%) in the HbAA group (p=0.235). Also, the prevalence of I431V mutation 48(64.0%) was higher in HbAA group than in SCA group 34(47.9%) (p=0.050). The predominant pfdhps haplotypes of parasites infecting children in both study groups were the single mutant haplotype: ISGKAA and the quintuple mutant haplotype: VAGKGS. In the SCA group, the prevalence of ISGKAA pfdhps mutant haplotype: 25(35.2%) was statistically significantly higher than 16(21.3%) found in HbAA group (p=0.032). In the HbAA group, the prevalence of VAGKGS pfdhps mutant haplotype 43(57.3%) was statistically significantly higher than 18(25.4%) found in SCA group (p < 0.001). There was no statistical significant difference in the prevalence of the other mutant haplotypes between both groups (Figure 2).
Prevalence of pfdhps mutant haplotype and pfdhps point mutations and their association with the degree of parasitaemia (GMPD)
The Geometric Mean Parasite Density (GMPD) was higher in all samples with pfdhps point mutations compared to those without each of the mutations; but the GMPD was only significantly higher in children with pfdhps A581G (2184.24 ± 12.57) and A613S (2143.88 ± 11.19) mutations than in those without A581G and A613S mutations (p=0.008 and p=0.012) respectively. The GMPD (2764.39±13.12) was only significantly higher in samples with VAGKGS mutations compared to that (846.45±6.46) in those without VAGKGS haplotype (p=0.003) (Table 2). On the contrary, the GMPD was significantly higher in samples without the ISGKAA mutant haplotype (1942.67±10.99) compared to that (694.54±10.99) in those with ISGKAA haplotype (p=0.016). The GMPD differed significantly across samples with the different mutant haplotypes (p=0.016); being highest in those with the quintuple mutant haplotype VAGKGS (2764.39±13.12) (Annex 2). The post hoc test showed that the GMPD in those with pfdhps quintuple mutant (VAGKGS) haplotype was significantly higher than (694.54 ± 10.99) in those with pfdhps single mutant haplotypes (ISGKAA).
Multiple binary logistic regressions model for the effect of degree of parasitaemia (geometric mean parasite density) and haemoglobin phenotype on the prevalence of pfdhps point mutations/haplotypes
Multiple logistic regressions model was constructed for the effect of parasite density and haemoglobin phenotype on the presence of pfdhps point mutations and pfdhps mutant haplotypes. HbAA phenotype was the only significant predictor for the presence of A581G (aOR 2.3, 95% CI: 1.105 to 4.851; p=0.026) and A613S point mutations (aOR 2.7, 95% CI: 1.293 to 5.745; p= 0.008). Parasite density was not a significant predictor for A581G and A613S point mutations (Table 3). Similarly, a significant relationship was found between HbAA phenotype and VAGKGS haplotype, which is three (3) times more likely to occur in persons with HbAA phenotype (aOR 3.0, 95% CI: 1.375 to 6.499; p=0.006) (Table 3).
An important finding in this study is the distinct pattern and prevalence of the pfdhps point mutations: I431V, S436A, A437G, A581G, and A613S and mutant haplotypes identified in this study; the prevalence of each point mutation was higher in children with HbAA compared to those with SCA. The predominant pfdhps mutant haplotypes found in this study were VAGKGS (which was significantly higher in children with HbAA) and ISGKAA (which was significantly higher in children with SCA). Haemoglobin phenotype AA was a significant predictor for the occurrence of VAGKGS. The presence of the emerging pfdhps I431V, and the high prevalence of pfdhps A581G and A613S mutations found in both children with SCA and those with HbAA were distinct in this study. The I431V is an emerging mutation that was first described by Sutherland et al., in 2009, in isolates from British travellers, who visited Nigeria [14]. The I431V mutation has also been reported in other studies [6,15,22,23]. The similar prevalence of A581G, A613S and I431V mutations found in this study was also demonstrated in the study by Sutherland et al. 2009 [14]. The prevalence of the A437G mutation was the highest, with almost 100% prevalence in both study groups. A similar high prevalence was seen in Angola, Equitorial Guinea and Cameroon with a prevalence of up to 97.9%, 90.5% and 90% respectively [24-26]. This high prevalence may be due to the fact that the A437G mutation is the first point mutation to occur in pfdhps gene, and overtime this mutation has become fixed and stable in Africa.
Another important finding in this study was the complete absence of the pfdhps K540E mutation and the double mutant haplotype (A437G+K540E) in children with SCA and HbAA. This finding is in keeping with other studies done in West Africa, with low prevalence of K540E in children [27,28]. On the contrary, in Nigeria, Happi et al. in Ibadan, in 2004, reported a K540E prevalence of up to 24%. However, similar to our finding, Pearce et al. in 2009 in Nigeria, found a complete absence of the K540E mutation [29-30]. A K540E prevalence of 50% is the threshold above which the efficacy of SP as IPT in young children is compromised [11]. Therefore, the absence of the pfdhps K540E mutation in this study has significant implications that SP remains efficacious as IPT in infants and young children with SCA and those with HbAA. There were two major pfdhps mutant haplotypes found in this locale: the single mutant haplotype - ISGKAA and the quintuple mutant haplotype - VAGKGS. These mutant haplotypes were also the major mutant haplotypes described by Sutherland et al. 2009, Oguike et al., 2016 and Chao et al. 2020 in isolates from cases of imported malaria from Africa [6,14,15]. Similar to our study, the prevalence of the VAGKGS (80%) haplotype was higher than that of the ISGKAA haplotype (50%) found in the study by Sutherland et al. 2009 [14]. The fact that the prevalence of the pfdhps point mutations and mutant haplotype VAGKGS was high in this study, despite lack of direct exposure to SP in the study population (both those with SCA and HbAA), further confirms that the spread of antifolate resistance is not confined only to the population directly exposed to SP, but the entire population in a region where SP is used (such as in IPTp) is at risk of SP resistance [31].
The prevalence of pfdhps point mutations S436A, A581G and A613S were significantly higher in children with HbAA compared to those in children with SCA. This may mean that the homozygous sickle cell gene is associated with a lesser risk of selection or spread of these pfdhps point mutations compared to the normal homozygous Haemoglobin A gene. A multiple logistic regression model showed that the homozygous Haemoglobin A gene is a significant predictor of the presence of the pfdhps A613S mutation (Table 3). The prevalence of the quintuple mutant pfdhps haplotype (VAGKGS) was significantly higher in children with HbAA compared to that in children with SCA, while a significantly higher prevalence of the pfdhps single mutant haplotype (ISGKAA) was found in children with SCA compared to that in children with HbAA. In this study, multiple logistic regressions (Table 3) showed that HbAA was a significant predictor of the presence of the VAGKGS haplotype, which is 2.5 times as likely to occur in children with HbAA haplotype compared to those with SCA. Thus, it can be inferred that the normal haemoglobin AA allows for more selection of parasites with mutant pfdhps haplotype: VAGKGS (which indicates a high level of SP resistance), while the homozygous sickle cell gene protects against its selection.
More so, in this study, the GMPD in those with HbAA (3671.13±10.64) was significantly higher than (522.89±5.30) in those with SCA (t=-5.72, p < 0.001). According to the mechanism of spread of antimalarial resistance, resistant genes are transmitted in a population via gametocytes [31,32]. In children with SCA, spread of SP resistance may less likely occur, due to a lesser parasite biomass and gametocyte production, compared to those with HbAA. There was a significant relationship between mean parasite density and the type of pfdhps mutant haplotype in the study population; the highest GMPD was found in those with VAGKGS mutant haplotype, being significantly higher than the GMPD found in those with the single mutant haplotype: ISGKAA. This supports the mathemathical models of White et al. and Pongtavornpinyo et al. that the chance of selection and preferential survival of drug-resistant mutants from patients with high parasite density is greater than if a similar mutant arose in a person with much lower parasitaemia [16,17,31]. However, parasite density was not found to be a significant predictor for the occurrence of VAGKGS. This study has some limitations; it was done in a single locale (Benin City), thus it may be limited in describing the widespread distinct pattern and prevalence of the pfdhps mutations/mutant haplotypes in children with SCA and HbAA in Edo State and in Nigeria.
The K540E mutation which is an indicator of significant resistance to SP leading to poor efficacy of SP-IPT in young children was totally absent in this study. Thus, there may be no significant resistance to SP in the study population, especially in those with SCA. Therefore, the use of SP as IPT can be explored in children younger than five years with SCA living in Benin City. The normal haemoglobin haplotype (HbAA), is a significant predictor of the occurrence of the quintuple mutant pfdhps haplotype: VAGKGS; while the homozygous haemoglobin sickle cell gene may confer protection against the occurrence and spread of mutations on the pfdhps genes in children with SCA.
What is known about this topic
- Mutations on the Plasmodium falciparum dihydropteroate synthase (pfdhps) genes are the molecular markers of Plasmodium falciparum resistance to Sulphadoxine;
- Parasite densities/Biomass in persons with HbAA are higher than that in those with SCA;
- Mathematical models and theories show that resistant parasite strains are more likely to occur in infections with high parasite densities than in those with lower parasite densities.
What this study adds
- This is the first study to demonstrate the mathematical model that selection of mutant haplotypes is more likely to occur in infections with high parasite density than in those with low parasite burden;
- The HbAA predisposes to the occurrence of the VAGKGS haplotype, while the HbSS phenotype may protect against the occurrence of pfdhps point mutations and the VAGKGS haplotype;
- The K540E mutation was totally absent in all samples in both study population (HbAA and SCA), thus the use of SP-IPT as malaria chemoprevention in children with SCA can be explored in Benin City.
The authors declare no competing interests.
Conception and study design: Izehiuwa Gertrude Enato, Ayebo Evawere Sadoh, Okoeguale Michael Ibadin and Magdalene Erhieyouvbe Odunvbun. Data collection: Izehiuwa Gertrude Enato and Iriagbonse Iyabo Osaigbovo Data analysis and interpretation: Izehiuwa Gertrude Enato, Ayebo Evawere Sadoh, Okoeguale Michael Ibadin, Magdalene Erhieyouvbe Odunvbun and Iriagbonse Iyabo Osaigbovo. Manuscript drafting: Izehiuwa Gertrude Enato. Guarantor of the study: Izehiuwa Gertrude Enato. All authors approved final version of the manuscript.
We wish to acknowledge the study participants, their parents/guidance, and the hospital management where this study was carried out. The contributions of colleagues at the University of Benin/University of Benin Teaching Hospital, Nigeria, and those of the London School of Tropical Medicine and Hygiene, UK, are acknowledged. In particular, I would like to thank Prof Ehijie Enato of the Department of Clinical Pharmacy, University of Benin for his invaluable contributions to this work. The laboratory study at LSTMH was supported by the generous grant from Rosemary Weir Award.
Table 1: age and gender distribution of study population
Table 2: prevalence of pfdhps point mutations/mutant haplotypes and their association with geometric mean parasite density
Table 3: multiple binary logistic regressions model for the effect of degree of parasitaemia (geometric mean parasite density) and haemoglobin phenotype on the prevalence of pfdhps point mutations/haplotypes
Figure 1: prevalence of pfdhps point mutations in SCA and HbAA groups
Figure 2: prevalence of pfdhps haplotypes in SCA and HbAA groups
Annex 1: types of mutant pfdhps haplotype found in study population and the corresponding combination of point mutations (PDF-335KB)
Annex 2: mean parasite density in study participants with different pfdhps haplotype (PDF-330KB)
- Diop S, Soudré F, Seck M, Guèye YB, Diéye TN, Fall AO. Sickle-cell disease and malaria: evaluation of seasonal intermittent preventive treatment with sulphadoxine-pyrimethamine in Senegalese patients-a randomized placebo-controlled trial. Ann Haematol. 2011 Jan;90(1):23-7. PubMed | Google Scholar
- Dawam JA, Madaki JKA, Gambazai AA, Okpe ES, Lar-ndam N, Onu A et al. Monthly sulphadoxine-pyrimethamine combination versus daily proguanil for malaria chemoprophylaxis in sickle cell disease: a randomized controlled study at the Jos University Teaching Hospital. Nigerian Journal of Medicine. 2016 Apr-Jun;25(2):119-27. Google Scholar
- Triglia T, Menting JG, Wilson C, Cowman AF. Mutations in dihydropteroate synthase are responsible for sulphone and sulphonamide resistance in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA. 1997 Dec 9;94(25):13944-9. PubMed | Google Scholar
- Gregson A, Plowe CV. Mechanisms of resistance of malaria parasite to antifolates. Pharmacological reviews. 2005 Mar;57(1):117-45. PubMed | Google Scholar
- Enato IG, Sadoh AE, Ibadin OM, Odunvbun ME. Prevalence of Molecular Markers of Plasmodium Falciparum Resistance to Proguanil and Pyrimethamine in Children with Haemoglobin Phenotypes SS and AA in Benin City, Nigeria. West Afr J Med. 2021 Dec 30;38(12):1183-1189. PubMed | Google Scholar
- Oguike MC, Falade CO, Shu E, Enato IG, Watila I, Baba ES et al. Molecular Determinants of Sulfadoxine-Pyrimethamine Resistance in Plasmodium Falciparum in Nigeria and the Regional Emergence of DHPS 431V. Int J Parasitol Drugs Drug Resist. 2016 Dec;6(3):220-229 Epub 2016 Sep 29. PubMed | Google Scholar
- Wernsdorfer WH, Noedl H. Molecular markers for drug resistance in malaria: use in treatment, diagnosis and epidemiology. Curr Opin Infect Dis. 2003 Dec;16(6):553-8. PubMed | Google Scholar
- Brooks DR, Wang P, Read M, Watkins WM, Sims FG, Hyde JE. Sequence variation of the hydroxymethyldihydropterin pyrophosphokinase: dihydropteroate synthase gene in lines of the human malaria parasite, Plasmodium falciparum, with differing resistance to sulphadoxine. Eur J Biochem. 1994 Sep 1;224(2):397-405. PubMed | Google Scholar
- Plowe CV, Cortese JF, Djimde A, Nwanyanwu OC, Watkins WM, Winstanley PA et al. Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase and epidemiologic patterns of pyrimethamine-sulfadoxine use and resistance. J Infect Dis. 1997 Dec;176(6):1590-6. PubMed | Google Scholar
- Wang P, Read M, Sims PF, Hyde JE. Sulphadoxine resistance in the human malaria parasite Plasmodium falciparum is determined by mutations in dihydropteroate synthetase and an additional factor associated with folate utilization. Mol Microbiol. 1997 Mar;23(5):979-86. PubMed | Google Scholar
- World Health Organization. Intermittent preventive treatment for infants using sulphadoxine-pyrimethamine (SP-IPTi) for malaria control in Africa: Implementation Field Guide. September 20. Accessed January 28, 2022. PubMed | Google Scholar
- Gosling RD, Gesase S, Mosha JF. Protective efficacy and safety of three antimalarial regimens for intermittent preventive treatment for malaria in infants: a randomised placebo controlled trial. Lancet. 2009 Oct 31;374(9700):1521-32. PubMed | Google Scholar
- World Health Organization. Report of the technical consultation on Intermittent Preventive Treatment in Infants (IPTi). Technical expert group meeting on preventive chemotherapy 2009. WHO Headquarters, Geneva. Accessed 2 December, 2021.
- Sutherland CJ, Fifer H, Pearce RJ, Reza FB, Nicholas M, Haustein T et al. Novel pfdhps haplotypes among imported cases of Plasmodium falciparum malaria in the United Kingdom. Antimicrobial Agents Chemother. 2009 Aug;53(8):3405-10 Epub 2009 May 11. PubMed | Google Scholar
- Chao X, Hui S, Qingkuan W, Jin L, Ting X, Xiangli K et al. mutation profile of pfdhfr and pfdhps in plasmodium falciparum among returned chinese migrant workers. Antimicrobial agents and chemotherapy. 2019 Apr 25;63(5):e01927-18 Print 2019 May. PubMed | Google Scholar
- White NJ, Pongtavornpinyo W, Maude RJ, Saralamba S, Aguas R, Stepniewska K et al. Hyperparasitaemia and low dosing are an important source of anti-malarial drug resistance. Malar J. 2009 Nov 11;8:253. PubMed | Google Scholar
- Pongtavornpinyo W, Yeung S, Hastings IM, Dondorp AJ, Day NPJ, White NJ. Spread of anti-malarial drug resistance: Mathematical model with implications for ACT drug policies. Malar J. 2008 Nov 2;7:229. PubMed | Google Scholar
- Young MD, Coatney GR. In Human Malaria. Edited by FR. Moulton. Sci. 1941:25-29. PubMed | Google Scholar
- Jarra W. Malaria and the Red Cell. Edited by David Evered and Julie Whelan Pitman, London. 1983:137-152.
- Kirkwood BR, Sterne JAC. Essential medical statistics. Second edition. Blackwell publishing company. USA. 2003:413-428.
- Plowe CV, Djimde A, Bouare M, Doumbo O, Wellems TE. Pyrimethamine and Proguanil Resistance-conferring mutations in Plasmodium falciparum dihydrofolatereductase: Polymerase Chain Reaction Methods for Surveillance in Africa. Am J Trop Med Hyg. 1995 Jun;52(6):565-8. PubMed | Google Scholar
- Chauvin P, Menard S, Iriart X, Nsango SE, Tchioffo MT, Abate L et al. Prevalence of Plasmodium falciparum parasites resistant to sulfadoxine/pyrimethamine in pregnant women in Yaoundé, Cameroon: emergence of highly resistant pfdhfr/pfdhps alleles. J Antimicrob Chemother. 2015 Sep;70(9):2566-71. PubMed | Google Scholar
- Plowe CV. The evolution of drug-resistant malaria. Trans R Soc Trop Med Hyg. 2009 Apr;103 Suppl 1(Suppl 1):S11-4 Epub 2008 Dec 12. PubMed | Google Scholar
- Jiang T, Chen J, Fu H, Kai W, Yao Y, Eyi JUM et al. High prevalence of Pfdhfr-Pfdhps quadruple mutations associated with sulfadoxine-pyrimethamine resistance in Plasmodium falciparum isolates from Bioko Island, Equatorial Guinea. Malar J. 2019 Mar 26;18(1):101. PubMed | Google Scholar
- Tuedom AGB, Sarah-Matio EM, Moukoko CEE, Feufack-Donfack BL, Maffo CN, Bayibeki AN et al. Antimalarial drug resistance in the Central and Adamawa regions of Cameroon: Prevalence of mutations in P. falciparum crt, Pfmdr1, Pfdhfr and Pfdhps genes. PLoS ONE. 2021 Aug 19;16(8):e0256343. PubMed | Google Scholar
- Ebel ER, Reis F, Petrov DA, Beleza S. Historical trends and new surveillance of Plasmodium falciparum drug resistance markers in Angola. Malar J. 2021 Apr 7;20(1):175. PubMed | Google Scholar
- Mombo-Ngoma G, Oyakhirome S, Ord R, Gabor JJ, Greutelaers KC, Profanter K et al. High prevalence of dhfr triple mutant and correlation with high rates of sulphadoxine-pyrimethamine treatment failures in vivo in Gabonese children. Malaria J. 2011 May 14;10:123. PubMed | Google Scholar
- Duah NO, Quashie NB, Abuaku BK, Sebeny PJ, Kronmann KC, Koram KA. Surveillance of Molecular Markers of Plasmodium falciparum resistance to Sulphadoxine-Pyrimethamine 5 years after the change of malaria treatment policy in Ghana.Am J Trop Med Hyg. 2012 Dec;87(6):996-1003. PubMed | Google Scholar
- Happi CT, Gbotosho GO, Folarin AO, Akinboye DO, Yusuf BO, Ebong OO et al. Polymorphisms in Plasmodium falciparum dhfr and dhps genes and age related in vivo sulphadoxine-pyrimethamine resistance in malaria-infected patients from Nigeria. Acta Tropica. 2005 Sep;95(3):183-93. PubMed | Google Scholar
- Pearce RJ, Pota H, Evehe M-SB, Ba E-H, Mombo-Ngoma G, Malisa AL et al. Multiple Origins and Regional Dispersal of Resistant dhps in African Plasmodium falciparum Malaria. PLoS Med. 2009 Apr 14;6(4):e1000055 Epub 2009 Apr 14. PubMed | Google Scholar
- White NJ. Antimalarial drug resistance. J Clin Invest. 2004 Apr;113(8):1084-92. PubMed | Google Scholar
- Jeffery GM, Eyles DE. Infectivity to mosquitoes of Plasmodium falciparum as related to gametocyte density and duration of infection. Am J Trop Med Hyg. 1955 Sep;4(5):781-9. PubMed | Google Scholar