The effect of dexamethasone as an adjuvant in spinal anesthesia for femur upper extremity surgery: a prospective randomized trial
Ahlem Bousabbeh, Salma Ketata, Nizar Sahnoun, Mariem Keskes, Omar Ketata, Wiem Ben Amar, Imen Zouche, Abdelhamid Karoui
Corresponding author: Nizar Sahnoun, Department of Orthopedic Surgery, Habib Bourguiba University Hospital, 3000 Sfax, Tunisia 
Received: 29 Jun 2022 - Accepted: 05 Sep 2022 - Published: 19 Sep 2022
Domain: Drug delivery systems,Orthopedic surgery
Keywords: Spinal anesthesia, dexamethasone, sensory bloc, pain assessment
©Ahlem Bousabbeh et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Ahlem Bousabbeh et al. The effect of dexamethasone as an adjuvant in spinal anesthesia for femur upper extremity surgery: a prospective randomized trial. Pan African Medical Journal. 2022;43:29. [doi: 10.11604/pamj.2022.43.29.36117]
Available online at: https://www.panafrican-med-journal.com//content/article/43/29/full
Research 
The effect of dexamethasone as an adjuvant in spinal anesthesia for femur upper extremity surgery: a prospective randomized trial
The effect of dexamethasone as an adjuvant in spinal anesthesia for femur upper extremity surgery: a prospective randomized trial
Ahlem Bousabbeh1, ![]() Salma Ketata1,
Salma Ketata1, ![]() Nizar Sahnoun2,&, Mariem Keskes1, Omar Ketata2, Wiem Ben Amar3,
Nizar Sahnoun2,&, Mariem Keskes1, Omar Ketata2, Wiem Ben Amar3, ![]() Imen Zouche1, Abdelhamid Karoui1
Imen Zouche1, Abdelhamid Karoui1
&Corresponding author
Introduction: the aim of our study was to evaluate the efficacy of dexamethasone added to bupivacaine and sufentanilin spinal anesthesia to improve postoperative analgesiaafter femur upper extremity surgery.
Methods: we conducted a prospective controlled, randomized double-blinded clinical trial including patients proposed for surgery of the upper extremity of the femur under spinal anesthesia. The patients were randomly allocated to receive intrathecally 10 mg hyperbaric bupivacaine 0.5% with 5µg sufentaniland 2 ml normal saline (control group) or 10 mg hyperbaric bupivacaine 0.5% with 5 µg sufentanil and 8 mg dexamethasone (Dexa group). The patients were evaluated for onset time and duration of sensory block, duration of pain-free period, overage consumption of morphine in the 6 first postoperative hours, hemodynamic parameters, nausea, and vomiting, orother complications.
Results: fifty-eigth patients were analyzed. There were no significant differences in demographic data and onset time of the sensory block between the two groups. Sensory block duration was 121.55 ± 16.42 minutes in the control group and 183.62 ± 33.93 minutes in the Dexa group which was significantly higher in the Dexa group (P<0.001). The pain-free period was longer in the Dexa group than in the control group (P<0.001). There was a reduction in morphine consumption during the first 6 postoperative hours in the Dexa group against the control group (p=0.02). The frequency of complications was not different between the two groups.
Conclusion: the addition of intrathecal dexamethasone in spinal anesthesia improved the postoperative analgesia after femur upper extremity surgery.
Fractures of the upper extremity of the femur affect frequently elderly patients and are responsible for significant morbidity and mortality [1]. Optimal management of these lesions is decisive for the quality of functional results. The anaesthetic technique of choice is spinal anaesthesia (SA) [2]. However, its duration, as well as postoperative analgesia, remains relatively limited. The addition of intrathecal (IT) adjuvants to local anaesthetics to improve and prolong per and postoperative analgesia is increasingly used [3]. Dexamethasone has shown its efficacy and harmlessness as an adjuvant to local anaesthetics [4,5] but has not benefited from sufficient experimentation in spinal anaesthesia. The aim of the study was to evaluate the efficacy of the addition of dexamethasone to bupivacaine and sufentanil for spinal anaesthesia to improve postoperative analgesia in patients undergoing surgery on the upper extremity of the femur.
We conducted a prospective controlled double-blinded randomized clinical trial in the anaesthesia-intensive care unit involving patients proposed for surgery of the upper extremity of the femur under spinal anaesthesia (SA).
Study population
Inclusion criteria were: patients older than 40 years old (since the fracture of the upper extremity of the femur affects the elderly patients) with an American Society of Anesthesiologists (ASA) score of I or II, candidates for surgery of the upper extremity of the femur proposed for DHS (Dynamic Hip Scrue) under spinal anaesthesia (SA).
Non-inclusion criteria were: absolute contraindications to spinal anaesthesia, the patient's refusal to be included in the study, known allergy to dexamethasone or local anaesthetics, and misunderstanding of the visual analogue scale.
The exclusion criteria were: technical difficulties in performing SA, partial or total failure of the SA requiring conversion to General Anesthesia (GA), a severe complication of anaesthesia, or surgery requiring the conversion to general anaesthesia.
Sample size: since there was no clinical trial investigating the effect of intrathecal dexamethasone on reducing postoperative pain after fracture of the upper extremity of the femur, the sample size was calculated from a previous pre-survey of 20 patients, that we did, showing that the mean duration of sensory block in the dexa group was 185.50 ± 29.64, while mean sensory block in the control group was 119.43 ± 15.20 setting alpha at 5% and power of study 90%. Calculation according to these values produced a minimal sample size of 16 patients in each group. We then designated 30 patients per group to have sufficient numbers after possible exclusions.
Randomization and allocation
Patients were randomized automatically according to a sequence generated by the sealedenvelope website into two groups: The Dexa group involved patients who received intrathecal dexamethasone added to bupivacaine and sufentanil while the Control group included patients who received intrathecal normal saline added to bupivacaine and sufentanil.
Interventions
In the operating room, patients were monitored by an electro-cardioscope, pulse oximetry, and non-invasive blood pressure monitoring. An 18-Gauge vein needle was placed and patients received 10 cc/kg of 0.9% of isotonic saline. Spinal anaesthesia was performed in the sitting position at L4-L5 or L5-S1 intervertebral space through a midline approach using a 25-gauge Quincke spinal needle. Patients of the control group received 10 mg (2 ml) of 0.5% hyperbaric bupivacaine with 5 µg of sufentanil (1 ml) diluted in preservative-free normal saline (2 ml) and patients of the Dexa group received 10mg (2 ml) of 0.5% hyperbaric bupivacaine with 5 µg of sufentanil (1 ml) and 8 mg preservative-free dexamethasone (2 ml). Overall 5 ml volume was administrated intrathecally in each group. The dexamethasone used in this study was the UNIDEX©. To facilitate the double-blinding method, the intrathecal solution was prepared by the anesthesiologist who was responsible for the randomization and injected slowly by a second anesthesiologist who was blinded to the nature of the solution. After the spinal anaesthesia, patients who also didn't know the nature of the solution were kept in the supine position, and oxygen of 3 L/ min was given through a face mask. The sensory block defined by the patient´s inability to feel pain and not differentiate between hot and cold was evaluated by the thermal method using a compress soaked with ether every two minutes until the installation of a metameric level at D6 for the authorization of the surgery. If after 20 minutes the level is insufficient, conversion to general anaesthesia was performed and the patient was excluded from the study.
The onset time of the sensory block was defined from the time of injection of drugs into the intrathecal space to the peak of sensory block (highest dermatome level) and the duration of sensory block was defined from a peak of sensory block up to 4 sensory level regressions or when the patients feel pain in the field of surgery. Intraoperative hemodynamic parameters were collected every 20 minutes until the end of the procedure. After the surgery, the patient was transferred to the Post Interventional Monitoring Room (PIMR) for 6 hours where standard monitoring was established. Hemodynamic parameters, pain assessment, and side effects were collected. The sensory block was re-evaluated until total regression. For the pain assessment, we used the visual analogue pain scale (VAS) previously explained to the patient. Postoperative analgesia was provided by 1 g of paracetamol and 2 mg Morphine titration every 5 minutes if the VAS value was greater than 3. After that, patients were transferred to their referral department. They were examined every day before leaving the hospital and 1 month later and asked about any postoperative complications.
Data collection: pre- and intra- operative data were collected including patients´ demographics, ASA scores, and duration of surgery. We collected anaesthetic and analgesic parameters such as the onset time and the duration of the sensory block and pain-free period, VAS at the 2nd, 6th, 12th and 24th postoperative hours, the overage dose of morphine consumption, and the hemodynamic parameters (heart rate, systolic, diastolic arterial pressure) every 20 minutes for the first two postoperative hours then at the 2nd, 6th, 12th and 24th postoperative hours. The collection of post-operative data was done by a third anesthesiologist who didn´t know the randomized groups.
Variables: our primary outcome was the duration of the sensory block defined by the duration of inability to feel pain and not differentiate between hot and cold. Our secondary outcomes were the onset time of the sensory block, the pain-free period after surgery, the overage dose of morphine consumption for the first 6 postoperative hours, and the side effects related to SA and intrathecal dexamethasone (nausea, vomiting, hypotension, bradycardia, shivering, headache or dyspnea).
Statistical analysis: SPSS version 25.0 software was used for data analysis. We checked the normality of the distribution by the Shapiro-Wilk test for the quantitative variables. Continuous variables and data with a normal distribution are expressed as means (SD) and as medians with the semi-interquartile ranges (SIR) otherwise. Qualitative variables were expressed as frequency distributions. Univariate comparisons between the two groups of patients were performed using t- student test, Mann-Whitney test, and Pearson´s Chi-square test. Statistical significance was defined as p < 0.05.
Ethical considerations: this study was conducted after approval of the southern protection committee of people (C.P.P.SUD) under the aegis of the Health Ministry of the Tunisian republic reference CPP SUD N°0215/2020for the nature of the product and the conduct of the study. This study was conducted after the written informed consent of patients.
Finding statements: we had no financial support for this work.
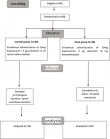
Sixty patients were included in our study and were divided into two equivalent groups. Two patients were excluded for a failure of spinal anaesthesia and a surgical duration longer than the spinal anaesthesia duration. The number of patients analyzed was 58 with 29 patients in each group (Figure 1).
General characteristics: our sample was characterized by a mean age = 71.21 ± 13.8 years, a sex ratio (male/female) =1.15 and a mean BMI = 28.775 ± 7.401 kg/m². There was no statistical difference between the Dexa and control groups in terms of demographic data, comorbidities, and duration of surgery (Table 1).
Postoperative analgesia: there was no significant difference in the mean of the onset time of the sensory block between the 2 groups. It was 5.62 ± 1.49 minutes for the Dexa group and 5.14 ± 1.02 minutes for the control group (P= 0.15). The duration of the sensory block was 121.55 ± 16.42 minutes in the control group and 183.62 ± 33.93 minutes in the Dexa group with a P-value less than 0.001. The pain-free period in the Dexa group was 273.1 ± 33.97 minutes was longer than that in the control group151.72 ± 27.78 with a P-value less than 0.001 (Table 2). The comparison of VAS (Visual Analogue Pain Scale) during the first 24 postoperative hours between the two groups showed a significant difference at all measurement points in favour of the Dexa group compared to the control group (Table 3). In our study, we showed a reduction in the morphine consumption during the first 6 postoperative hours in the Dexa group of 0.21 mg [0-4] mg against the control group of 1.8 mg [0-6] with p=0.02 (Table 2)
Side effects: there was no significant difference in heart frequency, systolic blood pressure, and diastolic blood pressure intra and the post-operative period between the two groups. No postoperative neurological deficit or allergic reactions were observed in any patients. There was no significant difference between the 2 groups in terms of other postoperative complications such as itching, nausea, and vomiting (Table 4).
Our study is one of the few studies in the literature to have used dexamethasone as an adjuvant to SA. We conducted a prospective double-blinded randomized clinical trial including patients proposed for upper femur surgery under spinal anaesthesia. Fifty-eight patients were analyzed and randomized into two groups of 29. This study showed that Dexamethasone significantly prolonged the analgesic effect of bupivacaine and sufentanil in spinal anaesthesia, without any effects on the onset time of the sensory block, or altering hemodynamic stability and without the occurrence of side effects. Several experiments demonstrated the analgesic effects of steroids in neuraxial and peripheral blocks.
Jabbari et al. [6], used dexamethasone in patients who had orthopaedic surgery under spinal anaesthesia. This study showed that the addition of intrathecal dexamethasone to bupivacaine significantly improved the duration of the sensory block and increased the time of the first analgesic intake without affecting the onset time of the sensory block. Sakis et al. [7], showed in a prospective randomized clinical trial involving 60 patients with ASA2 and ASA3 status, programmed for femur fracture, that the addition of dexamethasone to the anaesthetic improved the quality of the analgesia. Fayzal et al. [8] showed in a prospective study including 60 patients proposed for the cesarean section that the addition of intrathecal dexamethasone to bupivacaine hyperbaric significantly increased the duration of analgesia compared to bupivacaine hyperbaric.
Kotani et al. [9] administered methylprednisolone with bupivacaine intrathecally in patients with post-zosterian neuralgia. They concluded that this combination induced excellent and lasting analgesia. Several other studies have shown an extension of the duration of the sensory block in case of the addition of Dexamethasone to different local anaesthetics: a recent meta-analysis done in 2017 by Kyle Robert Kirkham et al. [10] including 33 clinical trials and 2138 patients, showed that the duration of the sensory and motor block of the brachial plexus was statistically significantly extended by Dexamethasone with a maximum dose of 4mg. Another meta-analysis from 2015, conducted by Albrecht et al. [11], covering 29 clinical trials and 1,659 patients, showed the same results. Several other studies [12-17] have demonstrated the analgesic prolongation of peripheral blocks of the upper limb following the addition of Dexamethasone.
In our study, we found that intrathecal dexamethasone does not increase the hypotension induced by local anaesthetics. This is consistent with the literature. Jabbari et al. [18], and El shourbagy et al. [19] showed in a study combining dexamethasone and bupivacaine in IT versus bupivacaine alone that there was no significant difference between the two groups in terms of hypotension episodes. In our study, no neurological complications were noted in both groups. Several studies in vitro or in vivo have evaluated the incidence of neurological complications related to the use of peri-nerve dexamethasone. No nerve damage was found in the various studies [20-22]. Moreover, a wide review of recent literature has not found any cases of neurotoxicity reported after perineural injection of dexamethasone as an adjuvant to the peripheral nerve block in 29 controlled trials involving 1,695 participants [11].
Limitation: it was impossible to operate on patients with the same surgeon and there is inter-individual variability in the pain perception. Future studies comparing the same dose of dexamethasone administered in IT and systemically will be necessary to recommend the use of dexamethasone in IT in daily clinical practice.
The addition of intrathecal dexamethasone to bupivacaine and sufentanil significantly improved the duration of sensory block in spinal anesthesia, increased the pain-free period, and decreased opioid requirements in postoperative management without side effects.
What is known about this topic
- Dexamethasone is a synthetic glucocorticoid;
- Dexamethasone, given intravenously, has analgesic and anti-inflammatory effects;
- Dexamethasone administered perineurally as an adjuvant to locoregional anesthesia significantly lengthens the duration of the sensory block.
What this study adds
- The addition of intrathecal dexamethasone to bupivacaine and sufentanil in spinal anesthesia: improved the duration of sensory block; increased the pain-free period; decreased opioid requirements in postoperative management; does not increase side effects related to spinal anesthesia.
The authors declare no competing interests.
Conception and design: Ahlem Bousabbeh, Salma Ketata, Nizar Sahnoun. Acquisition of data: Ahlem Bousabbeh, Salma Ketata, Nizar Sahnoun. Analysis and interpretation of data: Ahlem Bousabbeh, Salma Ketata, Nizar Sahnoun, Mariem Keskes, Omar Ketata, Imen Zouche. Drafting the article or revising it critically for important intellectual content: Ahlem Bousabbeh, Salma Ketata, Nizar Sahnoun, Mariem Keskes, Omar Ketata, Imen Zouche, Abdelhamid Karoui. Final approval of the version to be published: AhlemBousabbeh, Salma Ketata, Nizar Sahnoun, Mariem Keskes, Omar Ketata, Imen Zouche, Abdelhamid Karoui. All the authors have read and agreed in the final manuscript.
We would like to thank all the patients for their voluntary contribution to this study. We would also like to express our gratitude to the nurses and all the personnel of the operating unit, surgical ward, Orthopedic and anesthesiology departments of Habib Bourguiba University Hospital for their assistance and co-operation.
Table 1: comparison of demographic data between the 2 groups
Table 2: comparison of onset time and duration of sensory block, pain-free period and morphine consumption between the 2 groups
Table 3: comparison of VAS between the 2 groups
Table 4: comparison of side effects between the 2 groups
Figure 1: flow chart
- Noll E, Pottecher J, Diemunsch P. Anesthésie pour fracture de l´extrémité supérieure du fémur. Anesth Réanimation. Mars 2020;6(2):252-6. Google Scholar
- Tchouane S, Moyo JS, Binam F. La rachuanesthesie en orthopedie_traumatologie. MédecineAfr Noire. 1992;39(12):816-20. Google Scholar
- Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. Janv 2013;24(1):23-57. PubMed | Google Scholar
- Tandoc MN, Fan L, Kolesnikov S, Kruglov A, Nader ND. Adjuvant dexamethasone with bupivacaine prolongs the duration of interscalene block: a prospective randomized trial. J Anesth. Oct 2011;25(5):704-9. PubMed | Google Scholar
- Movafegh A, Razazian M, Hajimaohamadi F, Meysamie A. Dexamethasone added to lidocaine prolongs axillary brachial plexus blockade. Anesth Analg. Janv 2006;102(1):263-7. PubMed | Google Scholar
- Bani-Hashem N, Hassan-Nasab B, Pour EA, Maleh PA, Nabavi A, Jabbari A. Addition of intrathecal Dexamethasone to Bupivacaine for spinal anesthesia in orthopedic surgery. Saudi J Anaesth. Oct 2011;5(4):382-6. PubMed | Google Scholar
- Šakic L, Tonkovic D, Godan BJ, Šakic K. The influence of dexamethasone administration in spinal anesthesia for femur fracture on postoperative cognitive dysfunction. Period Biol. 10 juin 2015;117(2):281-5. Google Scholar
- Fayyaz MA, Khan AA, Ali RL. Comparison between Effect of Bupivacaine and Bupivacaine With Dexamethasone on Duration of Analgesia in Spinal Anaesthesia for Elective Caesarean Section. 2015;9(3):979-82. Google Scholar
- Kotani N, Kushikata T, Hashimoto H, Kimura F, Muraoka M, Yodono M et al. Intrathecal methylprednisolone for intractable postherpetic neuralgia. N Engl J Med. 23 nov 2000;343(21):1514-. PubMed | Google Scholar
- Kirkham KR, Jacot-Guillarmod A, Albrecht E. Optimal Dose of Perineural Dexamethasone to Prolong Analgesia After Brachial Plexus Blockade: a Systematic Review and Meta-analysis. Anesth Analg. janv 2018;126(1):270-9. PubMed | Google Scholar
- Albrecht E, Kern C, Kirkham KR. A systematic review and meta-analysis of perineural dexamethasone for peripheral nerve blocks. Anaesthesia. Janv 2015;70(1):71-83. PubMed | Google Scholar
- Chun EH, Kim YJ, Woo JH. Which is your choice for prolonging the analgesic duration of single-shot interscalene brachial blocks for arthroscopic shoulder surgerY intravenous dexamethasone 5 mg vs. perineural dexamethasone 5 mg randomized, controlled, clinical trial. Medicine (Baltimore). Juin 2016;95(23):1-6. PubMed | Google Scholar
- Cummings KC, Napierkowski DE, Parra-Sanchez I, Kurz A, Dalton JE, Brems JJ et al. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. Br J Anaesth. Sept 2011;107(3):446-53. PubMed | Google Scholar
- Holland D, Amadeo RJJ, Wolfe S, Girling L, Funk F, Collister M et al. Effect of dexamethasone dose and route on the duration of interscalene brachial plexus block for outpatient arthroscopic shoulder surgery: a randomized controlled trial. Can J Anaesth J Can Anesth. Janv 2018;65(1):34-45. PubMed | Google Scholar
- Huynh TM, Marret E, Bonnet F. Combination of dexamethasone and local anaesthetic solution in peripheral nerve blocks: A meta-analysis of randomised controlled trials. Eur J Anaesthesiol. Nov 2015;32(11):751-8. PubMed | Google Scholar
- Liu J, Richman KA, Grodofsky SR, Bhatt S, Huffman GR, Kelly JD, et al. Is there a dose response of dexamethasone as adjuvant for supraclavicular brachial plexus nerve block- A prospective randomized double-blinded clinical study. J Clin Anesth. Mai 2015;27(3):237-42. PubMed | Google Scholar
- Woo JH, Kim YJ, Kim DY, Cho S. Dose-dependency of dexamethasone on the analgesic effect of interscalene block for arthroscopic shoulder surgery using ropivacaine 0.5%: A randomised controlled trial. Eur J Anaesthesiol. sept 2015;32(9):650-5. PubMed | Google Scholar
- Jabbari A, Hassan-nasab B, Maleh P, Bani-hashem N, Pour E, Nabavi A. Addition of intrathecal Dexamethasone to Bupivacaine for spinal anesthesia in orthopedic surgery. Saudi J Anaesth. 2011;5(4):382. PubMed | Google Scholar
- El-Shourbagy M, Mammdouh A, Shawky M, Mohamed H. Addition of intrathecal dexamethasone to bupivacaine for spinal anesthesia in cesarean section. EvidBasedWomensHealth J. 1 mai 2019;9(2):416-24. Google Scholar
- Bennis M. Toxicité périneurale de la dexaméthasone: une étude in vivo sur un modèle animal [thèse]. Université Toulouse lll - Paul Sabatier. 2015.
- Le Gaillard B. La toxicité périneurale de la dexamethasone in vivo: mythe ou réalité [exercice]. Université Toulouse III - Paul Sabatier. 2014.
- Williams BA, Hough KA, Tsui BYK, Ibinson JW, Gold MS, Gebhart GF. Neurotoxicity of adjuvants used in perineural anesthesia and analgesia in comparison with ropivacaine. Reg Anesth Pain Med. Juin 2011;36(3):225-30. PubMed | Google Scholar