Cardiovascular risk and stroke mortality in persons living with HIV: a longitudinal study in a hospital in Yaounde
Liliane Mfeukeu Kuate, Larissa Ange Kwangoua Tchuisseu, Ahmadou Musa Jingi, Charles Kouanfack, Francky Teddy Endomba, Christian Ngongang Ouankou, Leonard Ngarka, Jean Jacques Noubiap, Samuel Kingue, Alain Menanga, Pierre Ongolo Zogo
Corresponding author: Ahmadou Musa Jingi, Department of Clinical Medicine, Faculty of Health Sciences, The University of Bamenda, Bamenda, Cameroon 
Received: 19 Jul 2021 - Accepted: 16 Aug 2021 - Published: 02 Sep 2021
Domain: Cardiology,Diabetes care,Internal medicine
Keywords: Cardiovascular risk, HIV, stroke, death, Cameroon
©Liliane Mfeukeu Kuate et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Liliane Mfeukeu Kuate et al. Cardiovascular risk and stroke mortality in persons living with HIV: a longitudinal study in a hospital in Yaounde. Pan African Medical Journal. 2021;40:8. [doi: 10.11604/pamj.2021.40.8.30855]
Available online at: https://www.panafrican-med-journal.com//content/article/40/8/full
Research 
Cardiovascular risk and stroke mortality in persons living with HIV: a longitudinal study in a hospital in Yaounde
Cardiovascular risk and stroke mortality in persons living with HIV: a longitudinal study in a hospital in Yaounde
Liliane Mfeukeu Kuate1,2, Larissa Ange Kwangoua Tchuisseu1, ![]() Ahmadou Musa Jingi3,&,
Ahmadou Musa Jingi3,&, ![]() Charles Kouanfack4,
Charles Kouanfack4, ![]() Francky Teddy Endomba5, Christian Ngongang Ouankou6, Leonard Ngarka1,
Francky Teddy Endomba5, Christian Ngongang Ouankou6, Leonard Ngarka1, ![]() Jean Jacques Noubiap7, Samuel Kingue1, Alain Menanga1,
Jean Jacques Noubiap7, Samuel Kingue1, Alain Menanga1, ![]() Pierre Ongolo Zogo8
Pierre Ongolo Zogo8
&Corresponding author
Introduction: HIV infection is a well-known risk factor for stroke, especially in young adults. In Cameroon, there is a death of data on the outcome of stroke among persons living with HIV (PLWH). This study aimed to assess the cardiovascular risk profile and mortality in PLWH who had a stroke.
Methods: this was a retrospective cohort study of all PLWH aged ≥18 years admitted for stroke between January 2010 and December 2019 to the Cardiology Unit of the Yaoundé Central Hospital, Cameroon. Cardiovascular risk was estimated using the modified Framingham score, with subsequent dichotomization into low and intermediate/high risk. Mortality was assessed on day 7 during hospitalization (medical records), at one month, and one year by telephone call to a relative.
Results: a total of 43 PLWH who had a stroke were enrolled. Their mean age was 52.1 (standard deviation 12.9) years, most of them were female (69.8%, n = 30). There were 25 (58.1%) patients on concomitant antiretroviral therapy. The Framingham cardiovascular risk score at admission was low in 29 patients (67.4%) and intermediate to high in 14 patients (32.6%). Ischemic stroke was the most common type of stroke in 36 persons (83.7%). The length of hospital stay was 11.4 (interquartile range 9.2-13.7) days. Mortality at 1 year was 46.5% (n = 20).
Conclusion: stroke mortality was high in this population of PLWH. Most patients had a low Framingham score, suggesting that this risk estimation tool underestimates cardiovascular risk in PLWH.
The lifespan of persons living with HIV (PLHIV) has improved with the increased access to antiretroviral therapy (ART), thus reducing the occurrence of AIDS-related complications [1, 2]. On the other hand, as HIV becomes a chronic disease, the increased exposure to traditional cardiovascular risk factors raises the risk of atherosclerosis and its complications [3-5], particularly stroke [6]. Moreover, HIV is a risk factor for stroke [7]. Young adults, aged less than 49 years, have the highest risk of HIV infection, and they represent 10-20% of stroke victims [8, 9].
The pathophysiologic mechanisms explaining the occurrence of stroke in PLWH are not fully understood. However, HIV is thought to act directly through vasculitis or indirectly through opportunistic infections, cancers, coagulopathies, or cardioembolic diseases [10, 11]. In addition, HIV infection leads to chronic inflammatory and prothrombotic states that are the basis for cardiovascular diseases [10]. Furthermore, antiretroviral drugs increase the occurrence of dyslipidemia, insulin resistance and lengthen the time of exposure to traditional risk factors [10, 12, 13]. As a result, the incidence of stroke has been rising over the past decades [8].
In Cameroon, with the 90-90-90 targets, the ART coverage rate has increased [14]. In Douala, in 2019, HIV seroprevalence among patients hospitalized for stroke was estimated at 6.6%, representing one in 15 patients [15]. Very few data are available on post-stroke mortality in PLWH. These two conditions together represent a major problem, given the motor disability generated, as well as the heavy additional economic burden. In addition, the clinical picture of stroke in PLWH remains poorly characterized. To improve the prevention and primary management of stroke in PLHIV, we aimed to assess cardiovascular risk before the onset of stroke and to determine post-stroke mortality.
Study design and setting: this was a retrospective cohort study conducted at the Cardiology Unit of the Yaoundé Central Hospital (YCH), Cameroon. Located in the Centre region, it is one of the largest hospitals in the city of Yaoundé, with a high attendance rate, a varied technical platform, and good geographical accessibility. The YCH, is a referral hospital, who has an emergency, cardiology, a neurology and medical imaging unit where the multidisciplinary follow-up of stroke patients can be done. There is no stroke specialize unit, but patients were consulted and followed by the multidisciplinary team in the cardiology wards. We reviewed the medical records of patients admitted to the Cardiology Unit from 1 January 2010 to 31 December 2019. The one-year survival was assessed using phone calls and medical files.
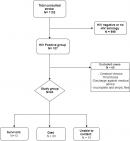
Study population: we included medical records of HIV-positive patients hospitalized for stroke using the WHO definition, confirmed by brain imaging (Computed Tomography [CT] or Magnetic resonance imaging [MRI]), and aged over 18 years. We excluded records of patients under 18 years of age, those without brain imaging (CT or MRI), those with cerebral venous thrombosis, unreadable or empty patient files, and those discharged against medical advice. Patients with unknown HIV status were also excluded (Figure 1).
Variables and data collection: data were collected from the complete records of cardiology admissions in a predefined data collection form
Exposure: regarding the exposure, the HIV patients were defined as each stroke patients who has already been diagnosed of HIV or who is under an HAART or who has been diagnosed during the hospitalization. All these elements were taken in the medical records. The characteristics of HIV-positive patients were duration since HIV diagnosis, opportunistic infections, and CD4 counts. Duration since HIV diagnosis was defined as the number of years since the HIV status discovery to stroke occurrence in years. Opportunistic infections were those noted in the file and concomitant with the cerebrovascular event. CD4 counts were those taken during hospitalization or in the 3 months before the stroke and marked in the record.
Covariates: socio-demographics, cardiovascular risk factors, laboratories and medical imaging results were abstracted from medical records. The socio-demographic factors included age and gender. The age in years was collected from the patient's file, corresponding to the age recorded at the time of the event. Given the lack of uniformity in the definition of 'young' or 'early stroke onset' and the resulting divergence of definitions, we define 'young' or 'early-onset' stroke as stroke occurring at less than 55 years of age in this study [16]. The cardiovascular risk factors included tobacco smoking, alcohol consumption, diabetes mellitus, and hypertension. Parameters on admission collected for this study included blood pressure, blood glucose, the Glasgow coma scale, and the modified NIH Stroke Score (NIHSS). We considered as PLWH any patient known with HIV, on ART or not; or any patient diagnosed with HIV during hospitalization. The cardiovascular risk score at admission was performed according to Framingham 2008 to assess the overall cardiovascular risk over 10 years. The variables used were age, gender, total cholesterol, HDL-c, treated and untreated systolic blood pressure, smoking, and diabetes. An algorithm based on validated predictors and coefficients (Framingham, 2008) was performed using SPSS software. Cholesterol levels were set to the following values: for total cholesterol ? 4.1mmol/L and HDLc > 1.6 mmol/L. The variables were recoded and classified into 2 modalities: low and moderate to high. Where low risk was defined as raising FRS < 10%, moderate to high risk was FRS ? 20%.The diagnosis of ischemic or hemorrhagic stroke was marked in the record. Ischemic stroke locations were grouped into sylvian (total sylvian, superficial or deep), anterior, and vertebrobasilar (posterior, basilar stem). For hemorrhagic strokes, the locations were classified as deep hemispheric (damage to the grey nuclei: thalamus, caudate nucleus, lenticulate nucleus, internal or external capsule), lobar (frontal, parietal, occipital, temporal) and sub tentorial (protuberance, cerebellum).
Stroke outcome: the duration of hospitalization was the number of days between admission and discharge. Mortality was assessed at 7 days, 1 month, and 1 year. Patients medical record were assessed to abstract the vital status at each stage. For 1-year mortality data, patients were contacted by telephone calls. These calls were made and repeated three times within two weeks. Non-reachable persons were considered as lost to follow-up. These were unavailable, unassigned numbers, or cases of people not recognizing the patient.
Statistical methods: data were analyzed using IBM SPSS Statistics version 23.0 for Windows (Chicago, Illinois, USA). Continuous variables were described by the mean and standard deviation (SD) or median with interquartile range (IQR), and categorical variables using their frequencies and percentages. or median and interquartile range based on the distribution law. Missing data were replaced by values calculated by imputation to the mean when the percentage of these was less than 5%.
Ethical consideration: this study was approved by the Ethics Committee of the Faculty of Medicine and Biomedical Sciences. Data were collected anonymously from medical records. For the patients contacted by telephone, a favorable opinion was obtained after an explanation of the study.
General characteristics: between January 2010 and December 2019, 1102 patients were admitted for stroke, among which 107 were HIV positive. In this group, 43 HIV-positive patients were eligible and included in this study and 64 were excluded (Figure 1). After one year, the vital status was assessed and 20 patients were dead. The mean age at stroke onset was 52.1 (SD 12.9) years. Most patients were female (69.8%, n = 30). The antiretroviral coverage rate was 58.1% (n = 25). Fifteen patients had opportunistic infections diagnosed, among them ten (23.3%) had cerebral cryptococcosis, two (4.7%) had probable bifocal tuberculosis, two (4.7%) cerebral toxoplasmosis, and one pulmonary pneumocystis (2.3%). Tenofovir-Lamivudine-Efavirenz was the most commonly used combination (56.0%, n = 24). The main socio-demographic characteristics are presented in Table 1.
Cardiovascular risk: on admission, the time of arrival at the hospital was late with a median of 3 days [1/2-6 days]. Several risk factors were found. Indeed, the majority of PLWH had a low Framingham cardiovascular risk (67.4%, n = 29) before admission. Table 2 summarizes the modified Framingham cardiovascular risk scores at admission and the clinical severity scores.
Brain imaging : regarding the type of stroke, 83.7% (n=36) of strokes were ischemic (Table 3). Among the cerebral ischemic strokes, the sylvian territory was the most affected in both groups 88.9% (n = 32). Furthermore, the deep hemispheric location was the most common in hemorrhagic strokes.
Mortality: despite this, the average length of hospital stay was 11.4 (SD 7.2) days with 27 patients (62.8%) experiencing complications during hospitalization. Infection was the most frequent complication. Infection was the most frequent intra-hospital complication. Mortality at 7 days was 14.0% (n = 6), increasing to 34.9% (n = 15) at 1 month and 46.5% (n = 20) at 1 year (Table 4).
This retrospective longitudinal study aimed to assess the cardiovascular risk of HIV-seropositive patients and their mortality after stroke. We obtained the following results. We found that the cardiovascular risk according to modified Framingham was low in the majority of patients (29 patients or 67.4%). Mortality was high and was markedly increasing over time, with about half of the patients who had died at one-year post-stroke. Age is a non-modifiable cardiovascular risk factor. The mean age at stroke onset in this study was 52.1 (SD 12.9) years. This mean is lower than that in the general Cameroonian population (58.7 years) [17]. This may suggest that stroke occurs in a younger population in HIV-positive people than in HIV-negative people. This result is similar to those obtained in Cameroon in 2019 (51.30 SD 10.37 years), and Tanzania 47.2 (SD 14.5)which find stroke in relatively young subjects [15, 18]. HIV-induced chronic inflammation and coagulation disorders increase their risk of stroke [10, 11], and may explain the early age at onset of cerebrovascular events. As a result, stroke in PLWH occurs in a young and active population and poses an economic cost problem.
The gender distribution of the population shows a predominance of women with 30 (69.8%). HIV prevalence among women in Cameroon is higher (3.4%) than among men (1.9%) [14]. In a hospital study in Cameroon, men were more likely to be found among PLWH with stroke [19]. In the literature, there is variability according to sex, with the majority of studies favoring a male preponderance [19-21]. Nevertheless, it is demonstrated that women living with HIV have a higher immune activation in than seronegative women and men [22, 23]. As well, they have an increased likelihood of developing non-calcified coronary plaques, which are biomarkers of atherosclerotic risk. Similarly, women living with HIV have an increased risk of ischemic stroke [24]. Consequently, HIV-positive women, as a group with a high risk of developing atherosclerosis complications, need to be carefully followed up. In addition, improved cardiovascular diseases prevention in HIV-positive women may decrease their risk of stroke.
Cardiovascular risk at admission was low in most patients. The assessment of cardiovascular risk in people living with HIV is ambiguous in the current literature [25-27]. As HIV is considered a vascular risk factor, it would be logical that the cardiovascular risk in people living with HIV would be increased [7]. In fact, on one hand, several studies have shown that conventional cardiovascular risk scores (Framingham, AHA/ACC) underrate cardiovascular risk in HIV/AIDS [25, 28-30]. However, on the other hand, other authors have shown that these standard scores are appropriate to assess cardiovascular risk, or overestimating the risk [26, 27]. For instance, in Cameroon, a study conducted by Noumegni et al. showed that the Framingham tool classified more HIV+ patients in the most at-risk group, while our study did not [27]. This discrepancy could be explained by the young age of our study population, with one-third of our sample under 45 years of age. Additionally, the high proportion of women who are known to have a lower score than men could contribute to the global low risk obtained. Moreover, none of our patients were on abacavir, indinavir, or lopinavir/r. The Framingham score does not include non-traditional risks factors in PLWH such as chronic inflammation, immune system activation and its pro-coagulant cascade, and HAART use [6, 31]. According to a Ugandan study, PLWH is twice more at risk of arterial stiffness [32]. Since antiretroviral drugs increase the rate of dyslipidemia and consequently the cardiovascular risk, it would therefore be advisable to construct a more adequate cardiovascular risk assessment score that includes both more sensitive biomarkers of inflammation (hsCRP), immunological markers, and antiretroviral treatment [33]. This relatively low cardiovascular risk in this study may also be attributed to the fact that PLWH is often attending clinics for routine check-ups, which usually include clinical and biological evaluation to detect cardiovascular risk factors at an earlier stage.
The performance of brain imaging is an important part of the management of stroke patients. We excluded cases with unmarked or inconclusive CT scans or MRI results. PLWH had high proportions of ischemic stroke. In the majority of studies performed, PLWH had more ischemic strokes [18, 20, 21, 34]. This result is similar to those found in Cameroon, Tanzania, and Malawi [15, 18, 20, 35]. Several factors would justify the occurrence of ischemic stroke at the expense of hemorrhagic stroke. HIV infection may predispose an individual to arterial and venous thrombosis due to protein C and protein S deficiencies. Certain infections including Mycobacterium tuberculosis (TB), Cryptococcus, Varicella zoster virus, and syphilis may be implicated because of the immunosuppression caused by HIV. Several reviews on stroke and HIV, advise actively searching for tuberculous meningitis and other opportunistic infections in PLWH who have had a stroke [10, 11]. Carotid locations (sylvian and anterior) were the most common in both groups. This result is similar to that found in a prevalence study with 70.8% of carotid locations for ischemic stroke in PLWH [15]. Therefore, in the case of ischemic stroke, infectious causes should be investigated. For hemorrhagic stroke, hypertension was the most frequent cause with 3 patients (42.9%). This is similar to the results found in Ghana, showing that hypertension was the main cause of cerebral hemorrhage in PLWH (85.7%) [36].
The duration of hospitalization was 11.4 (SD 7.2) days. This is longer than that found in the general Cameroonian population after a stroke (8.56 [SD 6.35] days). This result is similar to those found in the literature with a length of hospitalization of 9 (10-6), 10 (SD 8) days, and 10.3 (IQR 8.2,-12.5) days respectively in Cameroon, Thailand, and Tanzania [10, 13, 15]. The mortality rate at the end of the first 7 days was 14.0%, which is similar to the mortality rates in HIV-negative individuals as described by Dewan et al., but lower than that of Ekeh et al ( 26.7%) [37, 38]. This difference can be explained by the improved management of HIV-positive patients and increased surveillance of the occurrence of infections during their post-stroke follow-up. Indeed, the acute phase of stroke is a crucial period for survival and functional recovery. Thus, appropriate management can reduce the mortality rate during this period. Therefore, a good follow-up of PLWH is necessary.
The one-month mortality rates (34.9%) after stroke were similar to those in the general population in Nigeria (33.3%) and HIV-positive patients in Tanzania [18, 38]. Achieving similar mortality rates as the general population may indicate that HIV-positive patients have a unique protective effect. In fact, due to their age, they can recover faster from neurological damage and therefore have similar clinical severity. Moreover, no opportunistic infections were present in 65.1% of the patients and 58.1% were already on ART. These features may suggest that most of the patients were immunocompetent and therefore similar to the general population. Several studies have addressed this issue, but the conclusion is mixed. Indeed, in a study of the Thai national database, which did not include whether HIV-positive patients were on ART, the work described a high in-hospital mortality rate among PLWH, as well as numerous complications [21]. However, this mortality rate is still higher and may be due to inadequate emergency management. Indeed, the use of rt-PA in acute ischemic stroke is not available in our study site, and neurosurgical management for hemorrhagic stroke remains costly for patients in a developing country. Improved access to both financial and material resources for these innovative techniques could significantly reduce mortality, bearing in mind that the most affected population is made up of workers (active people) who are sources of household income.
As follow-up after stroke is an important aspect, several studies have evaluated mortality after one year. Indeed, in this sample, it was 46.5%, supporting those found in Malawi in 2012 with 40.1% mortality in PLWH [20]. This Malawian study described there were no significantly difference between PLWH and HIV negative person one year after stroke occurred. However, these figures are elevated compared to those of the general population in the world [8], in Africa [39], and in Cameroon [40]. The global burden of diseases estimated at 36.2% the stroke mortality rate in the world, while an African systematic review estimated it at 33.2 (95% CI: 23.6-44.5) [8, 39]. Additionally, in Cameroon, in two referral hospitals, a study reported 32.7% of deaths at one year in 2015 [40]. This high mortality rate can be due to our socio-economic context where the health insurance system is poorly implemented and the entire financial burden of stroke relies on victims and their families. Hence, access to rehabilitation, home care and regular medical checkup constitute an additional expense for the affected households. Consequently, there is a serious need of poststroke rehabilitation, since the majority of patients may not have access to acute therapy and may have at the end severe disability.
Moreover, in our study population, which is predominantly under 55 years, ischemic stroke is most common, the overall mortality remains high compared to other studies [41, 42]. In fact, Varona et al. in 2011, found about 4-5% of mortality among young people a year after an ischemic stroke, while Loes et al. in the Netherlands, noticed 2.4% [41, 42]. This difference may be due to better monitoring, reduction and management of risk factors in high-income countries compared to our study setting. Thus, identifying the risk factors of death in PLWH would enable us to take steps to improve treatment, survival, quality of life, and return to work in our context.
This study has several limitations. First, due to the use of medical records for the study, the inclusion of cases was limited to the files of patients who were already diagnosed or were diagnosed during the hospitalization of HIV infection. Thus, some patients who were not included might have been HIV-positive but not diagnosed. Similarly, given the health care cost associated with stroke, some patients may have been under-diagnosed due to failure to perform or unavailaibility of brain imaging and other tests requested. Moreover, by its retrospective nature, the absence, nonexistence, and poor representativeness of the archives for certain periods have reduced our sample size of the study knowing that the way in which they were written and store varied from one year to another. Indeed, the archives are not computerized in the Cardiology Unit. In addition, recruitment was done only on hospitalized cases; we did not include patients who died before admission and those who remained at home, so we may have missed some cases, it was a hospital based study. Therefore, all these elements could explain our relatively small sample size. However, our study paved the way for future large-scale case studies on stroke in HIV patients.
Mortality was high in this population of PLWH, with half of them who had died a year after stroke. There is a need to improve stroke prevention and management in these patients. Furthermore, most of these patients with stroke had a low Framingham score, suggesting that this risk estimation tool underestimates cardiovascular risk in PLWH. Cardiovascular risk prediction could be improved by the addition of biomarkers to the current risk stratification schemes.
What is known about this topic
- HIV is an independent cardiovascular risk factor through chronic inflammation and antiretroviral therapy;
- Stroke in young subjects occurs in a population at risk for HIV.
What this study adds
- Stroke mortality is high in PLWH in our setting;
- This study provides a global overview and sets the stage for future stroke studies in PLWH, especially women;
- The Framingham score seems inadequate to assess cardiovascular risk in PLWH.
The authors declared no competing interests.
Liliane Mfeukeu Kuate and Larissa Ange Kwangoua Tchuisseu designed the study, collected, analysised, interpretated the data and drafting the manuscript. Liliane Mfeukeu Kuate, Larissa Ange Kwangoua Tchuisseu, Ahmadou Musa Jingi, Charles Kouanfack, Francky Teddy Endomba, Christian Ngongang Ouankou, Leonard Ngarka, Jean Jacques Noubiap, Samuel Kingue, Alain Menanga and Pierre Ongolo Zogo revised the manuscript. All authors read and approved the final version of the manuscript.
The authors thank their colleagues in the cardiology department of the Yaoundé Central Hospital for their contribution to the management of the patients and the realization of this work.
Table 1: characteristics of the population
Table 2: distribution of Framingham score according to HIV serology
Table 3: distribution of stroke type and localization according to HIV serology
Table 4: distribution of in-hospital complications and mortality in the first 7 days, at 1 month, and 1 year
Figure 1: research processflow-chart
- Teeraananchai S, Kerr SJ, Amin J, Ruxrungtham K, Law MG. Life expectancy of HIV-positive people after starting combination antiretroviral therapy: a meta-analysis. HIV Med. 2017;18(4):256-266. PubMed | Google Scholar
- Wandeler G, Johnson LF, Egger M. Trends in life expectancy of HIV-positive adults on antiretroviral therapy across the globe: comparisons with general population. Curr Opin HIV AIDS. 2016;11(5):492-500. PubMed | Google Scholar
- Bigna JJ, Ndoadoumgue AL, Nansseu JR, Tochie JN, Nyaga UF, Nkeck JR et al. Global burden of hypertension among people living with HIV in the era of increased life expectancy: a systematic review and meta-analysis. J Hypertens. 2020;38(9):1659-1668. PubMed | Google Scholar
- Bigna JJ, Noubiap JJ. Global burden of hypertension in people living with HIV. BMC Med.2021;19(1):112. PubMed | Google Scholar
- Nansseu JR, Tounouga DN, Noubiap JJ, Bigna JJ. Changes in smoking patterns after HIV diagnosis or antiretroviral treatment initiation: a global systematic review and meta-analysis. Infect Dis Poverty. 2020;9(1):35. PubMed | Google Scholar
- Sun D, Wu Y, Yuan Y, Wang Y, Liu W, Yang J. Is the atherosclerotic process accentuated under conditions of HIV infection, antiretroviral therapy, and protease inhibitor exposure?: meta-analysis of the markers of arterial structure and function. Atherosclerosis. 2015;242(1):109-16. PubMed | Google Scholar
- Gutierrez J, Albuquerque ALA, Falzon L. HIV infection as vascular risk: a systematic review of the literature and meta-analysis. PLoS One. 2017;12(5):e0176686. PubMed | Google Scholar
- GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(5):439-458. PubMed | Google Scholar
- UNAIDS. UNAIDS DATA 2020- Joint United Nations Programme on HIV/AIDS. 2020th ed. 28-116.
- Benjamin LA, Bryer A, Emsley HC, Khoo S, Solomon T, Connor MD. HIV infection and stroke: current perspectives and future directions. Lancet Neurol. 2012;11(10):878-90. PubMed | Google Scholar
- 11.Singer EJ, Valdes-Sueiras M, Commins DL, Yong W, Carlson M. HIV stroke risk: evidence and implications. Ther Adv Chronic Dis. 2013;4(2):61-70. PubMed | Google Scholar
- Noubiap JJ, Bigna JJ, Nansseu JR, Nyaga UF, Balti EV, Echouffo-Tcheugui JB et al. Prevalence of dyslipidaemia among adults in Africa: a systematic review and meta-analysis. Lancet Glob Health. 2018;6(9):e998-e1007. PubMed | Google Scholar
- Nansseu JR, Bigna JJ, Kaze AD, Noubiap JJ. Incidence and risk factors for prediabetes and diabetes mellitus among HIV-infected adults on antiretroviral therapy: a systematic review and meta-analysis. Epidemiology. 2018;29(3):431-441. PubMed | Google Scholar
- Enquête Démographique et de Santé 2018. 2020th ed. Institut National de la Statistique. 363-384.
- Mapoure Njankouo Y, Mondomobe Atchom C, Halle MP, Mbatchou Ngahane BH, Luma NH. Prevalence of HIV infection among stroke patients in Douala. Med Sante Trop. 2019;29(2):184-189. PubMed | Google Scholar
- Putaala J. Ischemic stroke in young Adults. Continuum (Minneap Minn). 2020;26(2):386-414. PubMed | Google Scholar
- Mapoure YN, Kuate C, Tchaleu CB, Mbatchou Ngahane HB, Mounjouopou GN, Ba H et al. Stroke epidemiology in Douala: three years prospective study in a Teaching Hospital in Cameroon. World Journal of Neuroscience. 2014;4(5):406-414. Google Scholar
- Mlay M, Bakari M. The prevalence of HIV among patients admitted with stroke at the Muhimbili National Hospital, Dar es Salaam, Tanzania. Tanzania J Hlth Res.2010; 12(2):105-113. Google Scholar
- Mapoure YN, Mondomobe CAA, Nkouonlack C, Ayeah CM, Luma HN, Njamnshi AK. HIV infection does not influence stroke outcomes in HIV-infected patients: a prospective study. Rev Neurol (Paris). 2019;175(5):313-318. PubMed | Google Scholar
- Heikinheimo T, Chimbayo D, Kumwenda JJ, Kampondeni S, Allain TJ. Stroke outcomes in Malawi, a country with high prevalence of HIV: a prospective follow-up study. PLoS One. 2012;7(3):e33765. PubMed | Google Scholar
- Cumming K, Tiamkao S, Kongbunkiat K, Clark AB, Bettencourt-Silva JH, Sawanyawisuth K et al. Impact of HIV on inpatient mortality and complications in stroke in Thailand: a national database study. Epidemiol Infect. 2017;145(6):1285-1291. PubMed | Google Scholar
- Fitch KV, Srinivasa S, Abbara S, Burdo TH, Williams KC, Eneh P et al. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. The Journal of infectious diseases. 2013;208(11):1737-1746. PubMed | Google Scholar
- Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15(8):955-9. PubMed | Google Scholar
- Chow FC, Regan S, Zanni MV, Looby SE, Bushnell CD, Meigs JB et al. Elevated ischemic stroke risk among women living with HIV infection. AIDS. 2018;32(1):59-67. PubMed | Google Scholar
- Triant VA, Perez J, Regan S, Massaro JM, Meigs JB, Grinspoon SK et al. Cardiovascular risk prediction functions underestimate risk in HIV infection. Circulation. 2018;137(21):2203-2214. PubMed | Google Scholar
- Krikke M, Hoogeveen RC, Hoepelman AI, Visseren FL, Arends JE. Cardiovascular risk prediction in HIV-infected patients: comparing the Framingham, atherosclerotic cardiovascular disease risk score (ASCVD), Systematic Coronary Risk Evaluation for the Netherlands (SCORE-NL) and Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) risk prediction models. HIV Med. 2016;17(4):289-97. PubMed | Google Scholar
- Noumegni SR, Ama VJM, Assah FK, Bigna JJ, Nansseu JR, Kameni JAM et al. Assessment of the agreement between the Framingham and DAD risk equations for estimating cardiovascular risk in adult Africans living with HIV infection: a cross-sectional study. Trop Dis Travel Med Vaccines. 2017;3:12. PubMed | Google Scholar
- Nyirenda M. Assessment of cardiovascular disease risks using Framingham risk scores (FRS) in HIV-positive and HIV-negative older adults in South Africa. Prev Med Rep. 2021;22:101352. PubMed | Google Scholar
- Mateen FJ, Post WS, Sacktor N, Abraham AG, Becker JT, Smith BR et al. Long-term predictive value of the Framingham risk score for stroke in HIV-positive vs HIV-negative men. Neurology. 2013;81(24):2094-102. PubMed | Google Scholar
- Markowicz S, Delforge M, Necsoi C, De Wit S. Cardiovascular risk evaluation of HIV-positive patients in a case-control study: comparison of the D:A:D and Framingham equations. J Int AIDS Soc. 2014;17(4 Suppl 3):19515. PubMed | Google Scholar
- Benjamin LA, Bryer A, Lucas S, Stanley A, Allain TJ, Joekes E et al. Arterial ischemic stroke in HIV: defining and classifying etiology for research studies. Neurol Neuroimmunol Neuroinflamm. 2016;3(4):e254. PubMed | Google Scholar
- Siedner MJ, Kim JH, Nakku RS, Hemphill L, Triant VA, Haberer JE et al. HIV infection and arterial stiffness among older-adults taking antiretroviral therapy in rural Uganda. AIDS. 2016;30(4):667-70. PubMed | Google Scholar
- So-Armah K, Freiberg MS. HIV and cardiovascular disease: update on clinical events, special populations, and novel biomarkers. Curr HIV/AIDS Rep. 2018;15(3):233-244. PubMed | Google Scholar
- van Rensburg JJ, Mudzi W, Ntsiea V. The differences in functional recovery between HIV-positive and HIV-negative stroke survivors. Turk J Phys Med Rehabil. 2018;64(4):314-321. PubMed | Google Scholar
- Benjamin LA, Corbett EL, Connor MD, Mzinganjira H, Kampondeni S, Choko A et al. HIV, antiretroviral treatment, hypertension, and stroke in Malawian adults: a case-control study. Neurology. 2016;86(4):324-33. PubMed | Google Scholar
- Sarfo FS, Opare-Sem O, Agyei M, Akassi J, Owusu D, Owolabi M et al. Risk factors for stroke occurrence in a low HIV endemic West African country: a case-control study. J Neurol Sci. 2018;395:8-16. PubMed | Google Scholar
- Dewan KR, Rana PV. A study of seven day mortality in acute ischemic stroke in a teaching hospital in Chitwan. J Nepal Health Res Counc. 2014;12(26):33-8. PubMed | Google Scholar
- Ekeh B, Ogunniyi A, Isamade E, Ekrikpo U. Stroke mortality and its predictors in a Nigerian teaching hospital. Afr Health Sci. 2015;15(1):74-81. PubMed | Google Scholar
- Adoukonou T, Kossi O, Fotso Mefo P, Agbétou M, Magne J, Gbaguidi G et al. Stroke case fatality in sub-Saharan Africa: systematic review and meta-analysis. Int J Stroke. 2021;1747493021990945. PubMed | Google Scholar
- Nkoke C, Lekoubou A, Balti E, Kengne AP. Stroke mortality and its determinants in a resource-limited setting: a prospective cohort study in Yaounde, Cameroon. J Neurol Sci. 2015;358(1-2):113-7. PubMed | Google Scholar
- Rutten-Jacobs LC, Arntz RM, Maaijwee NA, Schoonderwaldt HC, Dorresteijn LD, van Dijk EJ et al. Long-term mortality after stroke among adults aged 18 to 50 years. JAMA. 2013;309(11):1136-44. PubMed | Google Scholar
- Varona JF. Long-term prognosis of ischemic stroke in young adults. Stroke Res Treat. 2010;2011:879817. PubMed | Google Scholar