Pathological fracture on osteolytic lesion
Ousmane Traore, Mhamed Elyagoubi
Corresponding author: Ousmane Traore, Département de Radiologie, Faculté de Médecine et d'Odonto-Stomatologie (FMOS), Bamako, Mali 
Received: 22 Aug 2020 - Accepted: 12 Aug 2021 - Published: 30 Aug 2021
Domain: Haematology
Keywords: Pathological fracture, X-ray, computed tomography (CT)
©Ousmane Traore et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Ousmane Traore et al. Pathological fracture on osteolytic lesion. Pan African Medical Journal. 2021;39:280. [doi: 10.11604/pamj.2021.39.280.25695]
Available online at: https://www.panafrican-med-journal.com//content/article/39/280/full
Pathological fracture on osteolytic lesion
Ousmane Traore1,2,&, Mhamed Elyagoubi2
&Corresponding author
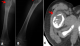
We report the case of a male child, aged three years, with no particular pathological history, who has presented for three months with isolated evasive lameness complicated two weeks before his admission by a total functional impotence of the right lower limb following a fall from his height. The clinical examination on admission found a child in good general condition, afebrile, with functional impairment of the right lower limb, associated with painful limitation of ipsilateral hip flexion. The blood count and C-reactive protein are normal. A frontal and lateral view of the right femur was performed (A, B) which revealed a proximal metaphyseal-diaphyseal lesion of the right femur, osteolytic (Ladwic type 2 geographic osteolysis), fairly well limited oval, containing thin partitions delimiting multiple cubicles. This gap blows the cortex which is partially ruptured on the external face of the bone with detachment of a thin cortical lamella intra-lesional testifying to a secondary fracture (clearly visible on the face X-ray). There are no other bone abnormalities or soft tissue detectable. A computed tomography (CT) scan of the right hip was prescribed as an adjunct and revealed an eccentric metaphyseal-diaphysealosteolytic lesion breaking the cortical without periosteal reaction (C).
Figure 1: A) frontal femur X-ray; B) lateral femur X-ray; C) right hip computed tomography (CT) scan: showing an eccentric osteolytic metaphyseal-diaphyseal lesion disrupting the cortex without periosteal reaction at the level of the right femur