Gastric duplication cyst with a red flag presentation: a case report
Dinesh Alagoo, Harivinthan Sellappan, Jayaprihyan Jayasilan, Nornazirah Azizan, Firdaus Hayati
Corresponding author: Firdaus Hayati, Department of Surgery, Faculty of Medicine and Health Sciences, Universiti Malaysia Sabah, Kota Kinabalu, Sabah, Malaysia 
Received: 18 May 2021 - Accepted: 06 Jun 2021 - Published: 18 Jun 2021
Domain: Gastroenterology,General surgery
Keywords: Duplication cysts, gastrointestinal bleeding, hypergastrinaemia, case report
©Dinesh Alagoo et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Dinesh Alagoo et al. Gastric duplication cyst with a red flag presentation: a case report. Pan African Medical Journal. 2021;39:141. [doi: 10.11604/pamj.2021.39.141.29895]
Available online at: https://www.panafrican-med-journal.com//content/article/39/141/full
Gastric duplication cyst with a red flag presentation: a case report
Dinesh Alagoo1, Harivinthan Sellappan1, Jayaprihyan Jayasilan1, Nornazirah Azizan2, ![]() Firdaus Hayati3,&
Firdaus Hayati3,&
&Corresponding author
Gastric duplication cyst (GDC) is a rare congenital malformation of the gastrointestinal (GI) tract. Despite being benign in the entity, its complications vary from an asymptomatic abdominal mass to fulminant or massive GI bleeding. A 28-year-old lady presented with unexplained GI haemorrhage, in which the upper endoscopy showed a classic feature of GI stromal tumour. The preoperative diagnosis was also confirmed by the computed tomography. She subsequently underwent surgical resection and the final histopathology was consistent with a benign entity of GDC.
Gastric duplication cyst (GDC), a rare congenital malformation, is typically associated with aberrations identified along the epithelial lining and through attachments to the alimentary tract. Frequently found in the ileum, oesophagus, and colon, while rarely present in the pharynx and tongue, duplication cyst is capable of occurring anywhere within the gastrointestinal (GI) tract [1]. Gastric duplication cyst comprises 4% of all GI duplications [2]. In close to half of the patients, congenital irregularities including alimentary tract duplications, oesophageal diverticulum, or spinal cord abnormalities were often detected [3]. The complications of GDC vary from an asymptomatic abdominal mass to fulminant or massive GI bleeding, and are most frequently diagnosed in young children. We present a 28-year-old lady, who was suspected to have a GI stromal tumour (GIST) through endoscopy but the final histopathology was consistent with a benign entity of GDC.
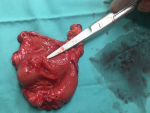
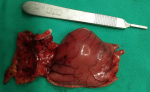
A 28-year-old lady, presented with a symptomatic anaemia due to an unexplained GI haemorrhage. She had two visits to the emergency department due to recurrent anaemic symptoms prior to her admission. She denied traditional medication usage or family history of malignancy. Clinically she was anaemic but not jaundiced. Examination of the abdomen revealed no mass. Her haemoglobin levels ranged between 8-9 g/dL (normal value: 10-12). She was then transfused with one pint of pack cells blood and was planned for an upper GI endoscopy. The oesophago-gastro-duodenoscopy was performed and showed a submucosal polypoidal mass at the greater curvature with a central umbilication that represents a GIST. There was no active bleeding from the lesion. Even though there was no other source of intragastric pathology, it was concluded that the anaemia was due to a bleeding GIST. This was solidified based on her computed tomography (CT) given that it was a pyloric submucosal lesion with no evidence of metastatic lesions. She was then electively subjected for a laparoscopy-assisted antrectomy and roux-en-y reconstruction. The surgery was uneventful. The gross specimen revealed to be a submucosal lesion with a central umbilication (Figure 1, Figure 2). There were no necrotic, haemorhagic or cystic areas visualized. The final histopathology, however, revealed to be a benign entity of GDC (Figure 3). Her colonoscopy then showed to have multiple sigmoid polyps, which turned out to be hyperplastic polyps. She is now on yearly follow up.
GDC is a peculiar congenital malformation. Though the pathophysiology of the congenital development is unknown, several mechanisms have been proposed including recanalization failure of the bowel lumen following the solid-epithelial phase of the intestinal development, persistence of epithelial outpunching in intestine, intestinal ischemia in early intrauterine life, and so on [4]. In majority, it is spherical non-communicating cysts. It rarely interacts with the chest wall, spleen, pancreatic duct, and the intrahepatic bile duct [5]. As in our case, it is a communicating type involving the stomach. What we noted in our case was in agreement with previous case reports where it was predominantly found in the greater or lesser curvature of the stomach [5].
In a majority of the patients, GDC is often detected in the first year. It is only diagnosed in adults upon an incidental finding or due to a complication [6]. The gastric outlet obstruction often leads to abdominal mass or vomiting and occasionally gastric ulcer, perforation, haemorrhage, abdominal pain, hemoptysis, pancreatitis, or hemobilia [6]. Based on past studies, GDC-associated massive GI haemorrhage was rarely detected. However, it is crucial to note that GDC is associated with different types of ulcers, including gastric, duodenal, or colonic, which cause internal bleeding, perforation, abdominal pain and penetration. In our case, we concluded that the source of anaemia is due to bleeding GDC as the endoscopy and radiologic investigations had ruled out other pathologies [7]. An important ulcer-precipitating factor, hypergastrinemia, is a unique association with GDC. However, serum gastrin levels were not measured in our patient.
In managing anaemia secondary to an occult bleeding, the sources of the bleeder must be identified. The initial step is to do an upper and followed by a lower endoscopy as it may be from the upper GI pathology [8]. If the bleeder arises from any gastric pathology as in this case, a complete surgical resection is the best treatment modality. By complete resection, it can lead to resolution of symptoms and elimination of potential malignancy [9]. Based on the endoscopic view, our patient showed a classic presentation of a GIST, hence it required a complete resection. Compared to the open approach, opting for a minimally invasive procedure allows for minimal stay in the hospital and lesser complications, while maintaining patient’s safety and efficacy.
GDC can manifest as a massive GI haemorrhage. It also can mimic endoscopic features of a GIST. Surgery is mandatory as it is symptomatic with histologic dilemma. Wherever possible, minimally invasive surgery is advisable.
The authors declare no competing interests.
Dinesh Alagoo and Jayaprihyan Jayasilan wrote the initial manuscript and involved in managing the patient. Harivinthan Sellappan acted as the main general surgeon in managing the patient and provided the literature review. Nornazirah Azizan provided the histologic figures and their descriptions. Firdaus Hayati became the correspondence and involved in final manuscript review. All authors read and approved the final manuscript.
We would like to thank the Director General of Health Malaysia for his permission to publish this article as a case report.
Figure 1: the gross appearance of the specimen showing that the tumor arises from the submucosal layer and appears to have a central umbilication
Figure 2: the serosal surface of the specimen showing no tumour breach
Figure 3: A) the histologic section denoting a surface foveolar epithelium of the gastric antral type mucosa (arrow) with underlying submucosal cyst (*). (Haematoxylin and eosin stain x4 magnification); B) another section showing multiple cystically dilated glands lined by gastric foveolar epithelium with higher magnification highlighting a single layer of cuboidal epithelium of the cyst with no nuclear atypia. (Haematoxylin and eosin stain x20 magnification)
- Tjendra Y, Lyapichev K, Henderson J, Rojas CP. Foregut duplication cyst of the stomach: a case report and review of the literature. Case Rep Pathol. 2016;2016:7318256. PubMed | Google Scholar
- Passos ID, Chatzoulis G, Milias K, Tzoi E, Christoforakis C, Spyridopoulos P. Gastric duplication cyst (GDC) associated with ectopic pancreas: case report and review of the literature. Int J Surg Case Rep. 2017;31:109-13. PubMed | Google Scholar
- D'Journo XB, Moutardier V, Turrini O, Guiramand J, Lelong B, Pesenti C et al. Gastric duplication in an adult mimicking mucinous cystadenoma of the pancreas. J Clin Pathol. 2004 Nov;57(11):1215-8. PubMed | Google Scholar
- Keeling JW, Khong TY. Fetal and Neonatal Pathology. London, UK. Springer. 2007;4th edition.
- Bhatti ZS, Anderson MA, Wasnik AP. Complete gastric duplication in an adult with associated anomalies. Clin Imaging. Mar-Apr 2016;40(2):244-6. PubMed | Google Scholar
- Rodríguez CR, Eire PF, Lopez GA, Alvarez EM, Sánchez FM. Asymptomatic gastric duplication in a child: report of a new case and review of the literature. Pediatr Surg Int. 2005 May;21(5):421-2. PubMed | Google Scholar
- Tanaka H, Masumoto K, Sasaki T, Sakamoto N, Gotoh C, Urita Y et al. Hypergastrinemia and a duodenal ulcer caused by gastric duplication. Surg Case Rep. 2016 Dec;2(1):75. PubMed | Google Scholar
- Azizan N, Hayati F, Zakaria AD, AbdShukor N. Intraductal papillary mucinous neoplasm presenting as bleeding duodenal mass: a surgical rarity. IMJM. 2019;18:123-6. Google Scholar
- Abbas A, Kermansaravi M, Daryabari SN, Shayanfar N. Laparoscopic resection of gastric duplication cyst containing gastrointestinal stromal tumour: a case report. J Minim Access Surg. 2021;17(1):98-100. PubMed | Google Scholar