Clinical profile and management of primary thyroid cancer in patients with nodular goitre at the Douala General Hospital, Cameroon
Edgar Mandeng Ma Linwa, Esthelle Minka Ngom, Georges Enownchong Enow Orock, Charlotte Eposse Ekoube, Esther Eleonore Ngo Linwa, Ngenge Michael Budzi, Martin Geh Meh, Richard Njock Louis
Corresponding author: Edgar Mandeng Ma Linwa, Faculty of Health Sciences, University of Buea, Buea, Cameroon 
Received: 18 Aug 2020 - Accepted: 08 Apr 2021 - Published: 28 Apr 2021
Domain: Histopathology,Pathology,Oncology
Keywords: Nodular goitre, thyroid, cancer, Cameroon
©Edgar Mandeng Ma Linwa et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Edgar Mandeng Ma Linwa et al. Clinical profile and management of primary thyroid cancer in patients with nodular goitre at the Douala General Hospital, Cameroon. Pan African Medical Journal. 2021;38:405. [doi: 10.11604/pamj.2021.38.405.25614]
Available online at: https://www.panafrican-med-journal.com//content/article/38/405/full
Research 
Clinical profile and management of primary thyroid cancer in patients with nodular goitre at the Douala General Hospital, Cameroon
Clinical profile and management of primary thyroid cancer in patients with nodular goitre at the Douala General Hospital, Cameroon
Edgar Mandeng Ma Linwa1,&, Esthelle Minka Ngom2, Georges Enownchong Enow Orock1, Charlotte Eposse Ekoube2, Esther Eleonore Ngo Linwa3, ![]() Ngenge Michael Budzi1, Martin Geh Meh1, Richard Njock Louis4
Ngenge Michael Budzi1, Martin Geh Meh1, Richard Njock Louis4
&Corresponding author
Introduction: thyroid cancer (TC) is considered to have become the fastest growing cancer in terms of incidence worldwide. Despite literature reporting a prevalence of 5-10% in clinically identified thyroid nodules, Cameroon still has limited data on the profile of TCs in patients with Nodular Goitres (NGs). The Objective were to describe the epidemiological, diagnostic and therapeutic profiles of TCs in patients with nodular goitres at the Douala General Hospital (DGH).
Methods: this was a retrospective cross-sectional analysis of patient records with diagnoses of NGs, over 11 years (2006 to 2016) at the DGH.
Results: overall, 187 patients (mean age= 46.8±13.9 years, men=27 (14.4%)) were included; 43 (23%) cancers were identified. The most common histological type was papillary cancer (50%). Nodule size of >4cm and hypoechogenicity were independently associated with malignancy. Most patients presented with TNM stage II (47.4%) and well-differentiated cancers were considered to be predominantly at low-risk according to MACIS (55%) and AMES (74%) scores. Surgery was offered to 95.3% of patients.
Conclusion: TCs are frequent in patients with NGs with papillary cancer dominating. A high index of suspicion should be held if a nodule is >4cm and/or is hypoechogenic. Prognostic studies are needed to describe the outcome of TCs in our setting.
A thyroid nodule is a discrete lesion within the thyroid gland that is radiologically distinct from the surrounding parenchyma [1]. Four to seven percent of the adult population has a palpable thyroid nodule [2]. The prevalence is much greater with the inclusion of nodules that are detected by ultrasonography (incidentalomas) or at autopsy. By the latter assessment, approximately 50 percent of 60-year-old persons have thyroid nodules [3]. Up to 5-20% of clinically identified nodules are malignant [4]. This corresponds to approximately 2 to 4 per 100,000 people per year, constituting only 1% of all cancers and 0.5% of all cancer deaths [5].
Thyroid cancer (TC) however, remains the most common malignant endocrine tumour worldwide and is considered to have become the fastest growing cancer in terms of incidence [3] and if recent trends are maintained, thyroid cancer may become the fourth most common cancer by 2030 in the United States [6]. In Saudi Arabia, it is the 2nd most common cancer in women and the 4th in men [7]. There are 5 main types of primary thyroid cancers: well differentiated (follicular and papillary), poorly differentiated (PDC), undifferentiated (anaplastic) and the sporadic cancers (medullary cancer). The classic treatment for TC is conventional thyroidectomy with adjuvant radioiodine ablation, and most patients can be cured with these treatments.
In Cameroon, thyroid incidentalomas were found to be very frequent with a prevalence of 28.3% and potential risk of malignancy in 12.9% [8]. Literature has demonstrated that mortality is higher in regions of endemic goitre because of more frequent advanced tumor stages at diagnosis and an increased ratio of more aggressive subtypes [9]. The Cameroon National Programme for the prevention and control of Iodine Deficiency was initiated in 1991 and was reported to be successful in reducing Iodine Deficiency Disorders [10]. However, few studies are available in our setting which determine the extent of thyroid nodules at presentation and demonstrate the characteristics that are associated with thyroid cancer. We therefore conducted this study with the following objectives: 1) determine the frequency and distribution of thyroid cancers in patients with nodular goitres; 2) compare nodular and clinical characteristics between benign and malignant nodular goitres; 3) compare the prognostic characteristics by age and gender in patients with thyroid cancers and 4. describe the management of patients with thyroid cancers.
Study design, period and setting: this was a hospital-based retrospective cross-sectional study at the ENT, Oncology/Radiotherapy, Pathology, and Endocrinology units of the Douala General Hospital (DGH) which is a third-level referral hospital in the Littoral Region of Cameroon. Records of patients were reviewed over a period of 11 years (2006 to 2016).
Participants and sampling: the study population was made up of patients who presented with a nodular goitre at any of the above units between 1st of January 2006 and 31st of December 2016. We included records of patients with sonographic and histopathological reports and excluded those diagnosed out of the study period, with incomplete records, inconclusive biopsy and/or secondary/metastatic cancers.
Study procedures and variables: data was collected using a pre-tested questionnaire. Clinical variables collected were age at diagnosis, gender, presence of palpable cervical lymph nodes. Nodular variables included size of thyroid nodule (in cm), echogenicity and location of nodule (on ultrasound). All considered predictor variables. Prognostic variables included the American Joint Cancer Committee (AJCC), Tumour Node Metastasis (TNM) stage, Metastasis Age at diagnosis Completeness if resection , local Invasion, Size of tumour (MACIS) and Age at diagnosis, Metastasis, Extent of tumour, Size of tumour (AMES) scores.
Data sources and definitions: only patients´ records were reviewed. Nodule size was considered as the largest diameter of a thyroid nodule as reported by the ultrasound report. In case of multinodular goitre, the size of the largest nodule evaluated was considered. Inconclusive biopsy was regarded as any biopsy result (apart from Bethesda II) that was not confirmed with excisional biopsy. Any record without age and sex was considered incomplete. Low-risk cancer was considered when MACIS score was less than 6. Patients´ records that lacked at least a report on cervical lymph node status and a chest X-ray or other metastatic work-up were considered not stageable.
Sample size considerations: using a pre-study prevalence estimate from South Africa (11,1%) [2], the minimum sample size (N) as determined by the Cochran´s formula,
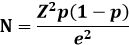 .
.
Where Z is the confidence level at 95% with a standard value 1.96, e is the allowable error of 5%, while p is the estimated prevalence according to a similar study of 11.1%, was 152 participants.
Data management and data analysis: the data collected was entered into and analyzed using SPSS version 20 statistical software. Quantitative variables were represented by their mean, standard deviation and range. Qualitative variables were represented as frequencies and percentages. Categorical variables were compared using the Chi-square or Fisher´s exact tests. The Student´s T-test was used to compare means. The level of statistical significance was set at p < 0.05.
Ethical considerations: ethical approval was granted by the Institutional Review Board of the Faculty of Health Sciences of the University of Buea. Administrative approval was obtained from the Director of the Douala General Hospital. Data entry forms were coded to ensure anonymity of patient personal information.
Socio-demographic characteristics of the study population: the ages of the patients at presentation ranged between 15 and 85 years with a mean age of 46.8 ± 13.9 years. The male to female ratio was 1:5.9.
Frequency and distribution of thyroid cancers in patients with nodular goitres: twenty three percent (n=43) of nodular goitres were malignant. Papillary cancer was the most common histological entity (50.0%) and this was followed by follicular cancer (40.5%) as shown in Figure 1.
Comparison of nodular and clinical characteristics between benign and malignant nodular goitres: the presence of cervical lymph nodes, nodule size >4cm, solitary thyroid nodules and hypoechogenicity were characteristics associated with malignancy as shown in Table 1. On multivariate analyses, using logistic regressions, we observed that hypoechogenicity (aOR= 5.6, 95% CI 1.3-23.9 p= 0.020) and nodule size >4cm (aOR= 23.2, 95% CI 4.6-177.2 p<0.001) were the only two factors independently associated with thyroid cancer. The details of these associations are shown in Table 2.
Comparison of the prognostic characteristics by age and gender in patients with thyroid cancers: follicular cancer was found to be the most common cancer in males, whereas, papillary cancer was the most frequent in females. Papillary cancers were more common in patients above 45 years whereas follicular cancers predominated in patients <45 years. One in every two females presented with Stage II disease. Patients > 45 years predominantly presented with Stage II while non-stageable disease was the most common in younger patients. Majority of patients were categorised as low-risk based on AMES and MACIS scores. All males were low-risk while upto 30.4% of the females were high risk according to AMES scores. Based on age, 62.5% and 37.5% of patients above 45years were low-risk based on AMES and MACIS scores respectively.
Management of patients with thyroid cancers: the most commonly practiced surgical treatment for males (80%) was total thyroidectomy only. It was however offered to only a few females (51.6%). Total thyroidectomy plus neck dissection was done in 20% of male versus 16.2% female patients. Thus, the most extensive surgical procedures were offered more to males. Total thyroidectomy plus neck dissection was offered in greater proportion (57.1% versus 42.9%) to patients > 45years. More males and patients aged < 45 years received chemotherapy and external beam radiotherapy. The observed differences were not statistically significant.
In this hospital-based study of medical records from 2006 to 2016, we found that one in five adults with nodular goitres had thyroid cancer. The majority were papillary cancers and factors associated with cancer were presence of palpable cervical lymph nodes, nodule size >4cm, solitary thyroid nodules and hypoechogenicity. Based on risk stratification systems, most patients presented with cancers that are considered low-risk cancers. In terms of management, the majority had total thyroidectomy alone offered.
Cancer prevalence as high as 38% has been reported in India, although regions like to Madagascar (22.3%) and Iran (28%) report prevalence similar to our study [11,12]. Though our study had a higher prevalence than that obtained in South Africa (11.1%) [2] the explanation may lie in the fact that, the latter study recruited all thyroid samples as opposed to only thyroid nodules as in our study. The most common histological type was papillary cancer as opposed to older studies [13,14] who found follicular cancer to be the most prevalent. This can be explained by the fact that these earlier studies were carried out in a context where most African countries were still considered iodine deficient areas. The distribution in our study correlates with recent studies carried out in Africa [15,16] and attests to the fact that Africa is gradually becoming an iodine sufficient area.
Though patients aged <45 years in our sample had a higher prevalence of malignancy, this was not statistically significant, as also demonstrated in studies from Korea and Brazil [17,18]. However, an association between malignancy, age <45 years [19] and age > 45 years [20] has been reported in other studies. The lack of association in our study can be explained by the much larger sample size used in the latter studies. Males are considered to be at higher risk of malignancy than females [2,20-22]. However, in agreement with few studies, [2,22] our study found no gender disparities. This may be explained by the relatively low frequency of males in our study. Despite the use of different evaluation methods [23-25], our study concurred with other studies in demonstrating an association between the presence of cervical lymph nodes and malignancy. Hypoechogenicity was present in up to 50% of patients and it was associated with malignancy. Other studies from Korea and Italy [17,26] denote a much lower prevalence of hypoechogenicity and refute any association with malignancy. This can be explained by the fact that these studies evaluated smaller nodules which predominantly display microcalcifications on ultrasound. Though literature [17,21] argues otherwise, nodules > 4cm were associated with malignancy and this in concordance with few hospital based studies conducted in Turkey and Brazil [18,23]. Our findings may be explained by our large mean nodule size of 4.13 cm. While some studies advocate MNG carry a higher risk of malignancy [18], others attribute the risk to sialyl-Thomsen-nouvelle (STN) [26]. In our study, STN was significantly associated with malignancy. Other studies however, refute any association [27,28]. These discrepancies can be explained by the few MNGs in our sample. On multivariate analysis, only nodule size and hypoechogenicity were independently associated with thyroid cancer.
While some studies have demonstrated that the more aggressive histologic subtypes have similar gender distribution [29], other studies have shown that men had a significantly higher proportion of aggressive tumours [30]. The latter findings were consistent with our study. Some studies report papillary cancer incidence to decrease with age whereas for all other sub-types, incidence rates generally increase with age. This is in discordance with our findings where the most aggressive subtypes predominated in patients <45 years, though these differences were not statistically significant. Studies report AJCC stage I or II disease to be the most common stage at presentation, findings similar to our study though with higher proportions than ours [31]. This signals a poorer prognosis in our setting and may be explained by earlier detection of cancers at screening. In our study, up to 74% and 55% of thyroid cancers were classified as low-risk according to AMES and MACIS scores respectively. This is lower than reports in western literature where more than 80.0% were low-risk on both risk stratification system [31,32] and this varied with age, contrary to our study.
Total thyroidectomy is the gold standard for patients with a preoperative diagnosis of papillary thyroid cancer when the nodule is greater than 1 cm in size. It is generally agreed upon that a therapeutic neck dissection should be pursued in the setting of well-differentiated thyroid cancer patients with clinically positive lymph nodes, whether in the central or lateral neck compartments [33]. In our study, total thyroidectomy only was the most commonly offered treatment modality and this management did not vary by age or gender. Our study had a number of limitations which must be mentioned. This was a single centre hospital based study which likely reflects the situation of the study hospital and so cannot be generalized to entire Cameroonian population. It was difficult to access all information from the records, so many records were excluded. The risk stratification scores used have neither been validated in our setting nor extensively worldwide. Finally, our sample size was small and hence might have failed to unearth certain associations or differences. However, this study is the first of its kind from Cameroon to explore and describe the clinical profiles of thyroid cancers in patients with nodular goiters in Cameroon, and hence will serve as a benchmark for future studies.
Thyroid cancers are frequent in patients with nodular goitres in our setting with papillary cancer being the most common subtype. Most patients present with low-risk cancers based on TNM, AMES and MACIS scores. The most common management is surgical. A high index of suspicion should be considered in patients with nodules more than 4cm and/or which are hypoechogenic. There is need for further research to be conducted in order to understand the risk factors, prognostic characteristics and outcome of thyroid cancers in our setting.
What is known about this topic
- In Cameroon, thyroid nodules have been shown to be frequent (28.3%) and 1/10 have potential risk of malignancy;
- In regions where goitre is endemic, mortality is higher and people consult at advanced tumor stages and have more aggressive subtypes. Ultimately, the clinical profile, cancer subtypes and prevalence of thyroid cancers depend on the prevalence of goitre in the population;
- After initiation of the iodization programme in Cameroun in 1991, few studies have been published to enable determine the impact of such programme on the profile and prevalence of thyroid cancers. Moreover, clinical characteristics that can enable practitioners rapidly identifying nodules with high risk of malignancy have not been determined.
What this study adds
- The prevalence of thyroid cancers amongst patients with thyroid nodules was found to be 23%;
- In this paper, we show that some clinical characteristics of thyroid nodules ( nodules more than 4cm and hypoechogenicity) give them a higher probability of being malignant. This is significant because it will help clinicians to have a higher index suspicion for cancer especially when considering settings where all histopathological workups are not always available and this will ultimately fasten management;
- The clinical profile of thyroid nodules described and the cancer subtypes found in this study could be a telltale sign of the effective prevention and control of Iodine deficiency disorders.
The authors declare no competing interests.
Edgar Mandeng Ma Linwa conceived the research questions, assisted with the study design and participant enrollment, designed the study protocol, collected the data, did data analysis and interpretation and wrote the manuscript. Edgar Mandeng Ma Linwa, Richard Njock Louis, Georges Enownchong Enow Orock, Charlotte Eposse Ekoube, Esther Eleonore Ngo Linwa, Ngenge Michael Budzi, Martin Geh Meh participated in revising the manuscript. Richard Njock Louis and Georges Enownchong Enow Orock supervised study implementation and reviewed both the protocol and the manuscript. All authors read and approved the final version of the manuscript.
We thank all the participants of this study.
Table 1: nodular characteristics in malignant and benign nodular goitres
Table 2:
multivariate regression analysis for independent risk factors
Figure 1: types of thyroid cancer
- Bryan Haugen R, Erik Alexander K, Keith Bible C, Gerard Doherty M, Susan Mandel J, Yuri Nikiforov E et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer.Thyroid. 2016 Jan;26(1):1-133. PubMed | Google Scholar
- Bhuiyan MMZU, Machowski A. Nodular thyroid disease and thyroid malignancy: experience at Polokwane Mankweng Hospital Complex, Limpopo Province, South Africa. South Afr Med J Suid-Afr Tydskr Vir Geneeskd. 2015;105(7):570-2. PubMed | Google Scholar
- Brito JP, Yarur AJ, Prokop LJ, McIver B, Murad MH, Montori VM. Prevalence of thyroid cancer in multinodular goiter versus single nodule: a systematic review and meta-analysis. Thyroid Off J Am Thyroid Assoc. 2013;23(4):449-55. PubMed | Google Scholar
- Wiyanto J, Kartamihardja AHS, Nugrahadi T. Can ultrasound predict malignancy in patient with thyroid cold nodule?. World J Nucl Med. 2016;15(3):179-83. PubMed | Google Scholar
- Anil G, Hegde A, Chong FHV. Thyroid nodules: risk stratification for malignancy with ultrasound and guided biopsy. Cancer Imaging. 2011;11(1):209-23. PubMed | Google Scholar
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, Liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-21. PubMed | Google Scholar
- Seada L, Hanan Oreiby, Ashraf Negm, Fawaz Al Rashid. A four - year study of thyroid carcinoma in hail region: increased incidence. World Acad Sci Eng Technol Int J Med Health Biomed Bioeng Pharm Eng. 2016;10(12):515-8. Google Scholar
- Moifo B, Moulion Tapouh JR, Dongmo Fomekong S, Djomou F, Manka´a Wankie E. Ultrasonographic prevalence and characteristics of non-palpable thyroid incidentalomas in a hospital-based population in a sub-Saharan country. BMC Med Imaging. 2017;17(1):21. PubMed | Google Scholar
- Lind P, Langsteger W, Molnar M, Gallowitsch HJ, Mikosch P, Gomez I. Epidemiology of thyroid diseases in iodine sufficiency. Thyroid. 1998;8(12):1179-1183. PubMed | Google Scholar
- Daniel Lantum. The conquest of iodine deficiency in Cameroon 1990-2007. J Cameroon Acad Sci. 2008;7(3):237-248. PubMed | Google Scholar
- Rakotoarisoa AH, Ralamboson SA, Rakotoarivelo RA, Raharisolo CV, Rakouth A, Ramiandrasoa AL et al. Thyroid cancers in Madagascar. Bull Soc Pathol Exot. 1990 2010;103(4):233-7. PubMed | Google Scholar
- Taghipour Zahir S, Binesh F, Mirouliaei M, Khajeh E, Noshad S. Malignancy risk assessment in patients with thyroid nodules using classification and regression trees. J Thyroid Res.2013;2013:983953. PubMed | Google Scholar
- Mulaudzi TV, Ramdial PK, Madiba TE, Callaghan RA. Thyroid carcinoma at King Edward VIII Hospital, Durban, South Africa. East Afr Med J. 2001;78(5):242-245. PubMed | Google Scholar
- Abdulkareem FB, Banjo AA, Elesha SO. Histological review of thyroid lesions: a 13-year retrospective study (1989-2001). Niger Postgrad Med J. 2005;12(3):210-4. PubMed | Google Scholar
- Djoumou F, Njock LN, Mindja Eko D, Vokwély Evehe E. Aspects épidemiologiques et histopathologiques des tumeurs thyroidïenne a Yaoundé. Rev Afr ORL Chir Cervico-Faciale. 2015;15(1).
- Solomon R, Iliyasu Y, Mohammed AZ. Histopathological pattern of thyroid lesions in Kano, Nigeria: a 10-year retrospective review (2002-2011). Niger J Basic Clin Sci. 2015;12(1):55. Google Scholar
- Choi YJ, Yun JS, Kim DH. Clinical and ultrasound features of cytology diagnosed follicular neoplasm. Endocr J. 2009;56(3):383-9. PubMed | Google Scholar
- Castro MR, Espiritu RP, Bahn RS, Henry MR, Gharib H, Caraballo PJ et al. Predictors of malignancy in patients with cytologically suspicious thyroid nodules. Thyroid. 2011;21(11):1191-8. PubMed | Google Scholar
- Rago T, Fiore E, Scutari M, Santini F, Coscio GD, Romani R et al. Male sex, single nodularity, and young age are associated with the risk of finding a papillary thyroid cancer on fine-needle aspiration cytology in a large series of patients with nodular thyroid disease. Eur J Endocrinol .2010;162(4):763-70. PubMed | Google Scholar
- Bessey LJ, Lai NBK, Coorough NE, Chen H, Sippel RS. The incidence of thyroid cancer by FNA varies by age and gender. J Surg Res. 2013;184(2):761-5. PubMed
- Tai J, Yang JL, Wu SC. Risk factors for malignancy in patients with solitary thyroid nodules and their impact on the management. J Cancer Res Ther. 2012;8(3):379-83. PubMed | Google Scholar
- Hanumanthappa MB, Gopinathan S, Rithin Suvarna, Guruprasad Rai, Gautham Shetty, Ashith Shetty et al. The incidence of malignancy in multi-nodular goitre: a prospective study at a tertiary academic centre. Journal of Clinical and Diagnostic Research.April 2012; 6(2):267-270. Google Scholar
- Kuru B, Gulcelik NE, Gulcelik MA, Dincer H. Predictive index for carcinoma of thyroid nodules and its integration with fine-needle aspiration cytology. Head Neck. 2009; 31(7):856-66. PubMed | Google Scholar
- Hands KE, Cervera A, Fowler LJ. Enlarged benign-appearing cervical lymph nodes by ultrasonography are associated with increased likelihood of cancer somewhere within the thyroid in patients undergoing thyroid nodule evaluation. Thyroid Off J Am Thyroid Assoc. 2010;20(8):857-62. PubMed | Google Scholar
- Jena A, Patnayak R, Prakash J, Sachan A, Suresh V, Lakshmi AY. Malignancy in solitary thyroid nodule: a clinicoradiopathological evaluation. Indian J Endocrinol Metab. 2015;19(4):498-503. PubMed | Google Scholar
- Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87(5):1941-6. PubMed | Google Scholar
- Barroeta JE, Wang H, Shiina N, Gupta PK, Livolsi VA, Baloch ZW. Is fine-needle aspiration (FNA) of multiple thyroid nodules justified? .Endocr Pathol. 2006;17(1):61-5. PubMed | Google Scholar
- Belfiore A, La Rosa GL, La Porta GA, Giuffrida D, Milazzo G, Lupo L et al. Cancer risk in patients with cold thyroid nodules: relevance of iodine intake, sex, age, and multinodularity. Am J Med. 1992;93(4):363-9. PubMed | Google Scholar
- Rahbari R, Zhang L, Kebebew E. Thyroid cancer gender disparity. Future Oncol Lond Engl. 2010;6(11):1771-9. PubMed | Google Scholar
- Price AJ, Ndom P, Atenguena E, Mambou Nouemssi JP, Ryder RW. Cancer care challenges in developing countries. Cancer. 2012;118(14):3627-35. PubMed | Google Scholar
- Yassa L, Cibas ES, Benson CB, Frates MC, Doubilet PM, Gawande AA et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer Cytopathol. 2007;111(6):508-16. PubMed | Google Scholar
- Hassanain M, Wexler M. Conservative management of well-differentiated thyroid cancer. Can J Surg. 2010;53(2):109-18. PubMed | Google Scholar
- Morrison SA, Suh H, Hodin RA. The surgical management of thyroid cancer. Rambam Maimonides Med J. 2014 Apr 28;5(2):e0008. PubMed | Google Scholar












