Febrile neutropenia following with single-low-dose methotrexate for the treatment of ectopic pregnancy: a case report
Burak Bayraktar
Corresponding author: Burak Bayraktar, Department of Obstetrics and Gynecology, University of Health Sciences Tepecik Training and Research Hospital, Izmir, Turkey 
Received: 19 Dec 2020 - Accepted: 27 Dec 2020 - Published: 07 Jan 2021
Domain: Gynecology,Obstetrics and gynecology
Keywords: Ectopic pregnancy, methotrexate, medical therapy, pharmacogenetics, case report
©Burak Bayraktar et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Burak Bayraktar et al. Febrile neutropenia following with single-low-dose methotrexate for the treatment of ectopic pregnancy: a case report. Pan African Medical Journal. 2021;38:17. [doi: 10.11604/pamj.2021.38.17.27507]
Available online at: https://www.panafrican-med-journal.com//content/article/38/17/full
Case report 
Febrile neutropenia following with single-low-dose methotrexate for the treatment of ectopic pregnancy: a case report
Febrile neutropenia following with single-low-dose methotrexate for the treatment of ectopic pregnancy: a case report
&Corresponding author
Methotrexate (MTX) is an effective, economical and safe drug used in the treatment of ectopic pregnancy. Complications are very rare. Herein, we reported a case of febrile neutropenia following single low-dose methotrexate for the treatment of ectopic pregnancy. Febrile neutropenia developed on day 4 of single-dose methotrexate administered intramuscularly. Although methotrexate single-dose regimen is quite effective and safe in ectopic pregnancy, febrile neutropenia can occur very rarely.
Ectopic pregnancy (EP) is defined as the placement of the fertilized ovum in a field outside the endometrial cavity. It is often seen in the tubal area, as well as in the hysterotomy scar, abdominal, ovarian, cervical, stump line, and rudimentary horn [1]. It accounts for 2% of all recognized pregnancies, and its frequency has been increasing worldwide in recent years [1]. At present, EP is a life-threatening condition and is still one of the most important causes of maternal loss in the first trimester [2].
EP can be treated medically or surgically. The choice of treatment varies depending on the clinical situation, EP localization, and available treatment options. Medical treatment has many advantages over surgical treatment in non-ruptured EP. These include less tubal damage, lower cost, and elevation in subsequent fertility potential. Methotrexate (MTX) is the most preferred agent in medical treatment for EP since the 1980s [1]. MTX is a folic antagonist that prevents deoxyribonucleic acid synthesis by inhibiting dihydrofolate reductase. It affects actively growing cells such as trophoblastic tissues, malignant cells, bone marrow, intestinal mucosa, and respiratory epithelium. The most important factor in the selection of treatment is a low side-effect and high-effectiveness profile. The most common side effects are stomatitis and conjunctivitis. Rare side effects include gastritis, enteritis, dermatitis, pneumonitis, alopecia, elevated liver enzymes, and bone marrow suppression.
For febrile neutropenia, fever by single oral measurement without any environmental factors is defined as temperature > 38.3°C or >38.0°sC for >1 h [3]. Neutropenia is defined as neutrophil level < 500/mm³ or neutrophil level between 500 and 1000/mm³ and is expected to fall <500/mm³ within 48 h [3]. Febrile neutropenia is extremely rare when using a single dose of MTX for EP. Herein, we present the case of febrile neutropenia due to the use of single-dose MTX in the treatment of EP.
A 29-year-old pregnant woman (gravida 2, para 1), without other disease and allergy history, presented with complaints of vaginal bleeding and menstrual delay. Based on her last menstrual period, she was 7 weeks pregnant. On gynecological examination, minimal vaginal bleeding was observed, and no additional pathology was noted. There was no defense or rebound in the abdominal examination. Other physical examination findings were heart rate of 72 beats/min and blood pressure of 110/70 mmHg. On transvaginal ultrasonography, the endometrium thickness was 6mm. The gestational sac or suspected mass was not observed in the cavity. The left adnexal area was normal. An 11mm diameter suspected mass was observed in the right adnexal area. The mass was compatible with gestational sac, but the yolk sac and fetal node could not be distinguished. Free fluid was not observed in the abdomen. The patient's beta human chorionic gonadotropin (β-hCG) value was 3155 mIU/mL. The patient was hospitalized with the diagnosis of EP. Her hemogram results were as follows: hemoglobin of 11 g/dL, hematocrit of 35.7%, platelet count of 192.000/mm³, leukocyte count of 7200/mm³, and neutrophil count of 4900/mm³. The patient's coagulation parameters (prothrombin time and international normalized ratio), liver enzymes (alanine aminotransferase, 10 IU/L; aspartate aminotransferase, 16 IU/L; lactate dehydrogenase, 480 IU/L), and creatine (0.8 mg/dL) levels were normal.
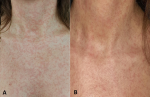
The patient was treated with a single dose of MTX. A total of 80-mg single-dose MTX was administered intramuscularly according to the 50 mg/m² dose calculation. On day 4 of treatment, generalized mild erythema and mucositis were observed (Figure 1). The patient's temperature was 39°C. On laboratory evaluation, the hemoglobin level was 10.5 g/dL, the hematocrit was 32.3%, and the platelet count was 174000/mm³, while the leukocyte count was 4100/mm³. The level of β-hCG decreased to 2420 mIU/mL. The neutrophil count of the patient was 700/mm³. Thereupon, neutropenic fever was diagnosed. Blood culture was taken from the patient, and empirical intravenous (IV) meropenem 3 x 500 mg and teicoplanin 1 x 400 mg were started. As a granulocyte colony-stimulating factor (CSF), filgrastim 30 million units/day was given subcutaneously, and calcium folinate 4 x 15 mg/day as an MTX antidote was started intravenously. On day 3 of treatment (day 7 of MTX treatment), the leukocyte count was 5700/mm³ and the neutrophil count was 2500/mm³, which began to rise. The patient did not need transfusion of blood products during follow-up. After 7 days of treatment (day 14 of MTX treatment), the patient's erythema and neutropenia improved and was monitored from the outpatient clinic (Table 1).
A single-dose MTX treatment is selected at the beginning of tubal EP because single-dose treatment is cheaper, requires less follow-up, does not require folic acid administration, and has fewer side effects with multiple-dose protocols. The rate of resolution reported in the literature is approximately 90% for single dose and multiple-dose regimen, but there is no meaningful difference [4]. In this protocol, MTX is administered at a dose of 50 mg/m2. In a single-dose regimen, follow-up is performed on day 4 and day 7 based on the β-hCG value. A 15% decrease in serum β-hCG concentration between day 4 and day 7 is an important indicator of treatment success [4].
MTX is a folic acid analog and binds to dihydrofolate reductase, reducing thymidylate level, purine synthesis, and cell proliferation. It also inhibits other folate-dependent enzymes such as 5-aminoimidazole-4-carboxamide ribonucleotide transformylase (AICAR) [5]. MTX can be administered orally, subcutaneously, intramuscularly, or intravenously [6]. Contraindications to MTX treatment are a history of severe hypersensitivity to MTX, alcoholism, alcoholic liver disease or other chronic liver disease, immunodeficiency syndromes (overt or laboratory evidence), and preexisting blood dyscrasias (e.g., bone marrow hypoplasia, leukopenia, thrombocytopenia, and significant anemia) [7]. Our patient did not have any of these contraindications.
MTX can cause rare toxicity if used at a low dose. The toxic effect can be divided into two groups: major and minor. Minor toxic effects of MTX are seen in 20%-30% and include headache, stomatitis, malaise, nausea, vomiting, diarrhea, and mild alopecia [8]. The major toxic effects of methotrexate are much less common in minor toxic effects but may be life-threatening. The major toxic effects of methotrexate can be listed as hepatic, renal, pulmonary and bone marrow disorders [8]. Myelosuppression and febrile neutropenia are very rare adverse effects of low-dose MTX. It can be overlooked because of its rarity. Untreated cases may lead to death. Some publications have indicated that methylene tetrahydrofolate reductase (MTHFR) polymorphism may be caused by low-dose MTX toxicity that occurs without a significant risk factor, such as impaired renal function [9, 10]. However, there is no consensus on the type of polymorphism and its evaluation before the first dose of MTX [11], so we did not perform polymorphism analysis in our patient.
Febrile neutropenia should be suspected if fever, generalized erythema, and mucositis are observed during single-dose MTX treatment. Hemogram should be taken quickly. Serial neutrophil follow-up should be carried out, blood culture should be taken, and empirical IV antibiotics should be started. Antibiotics to be given must have an antipseudomonal effect [12]. Calcium folinate is an MTX antidote and is easily available, so it should be administered. The addition of CSFs to treatment in febrile neutropenia reduces the duration of neutropenia, but does not have a positive effect on morbidity, fever duration, IV administration of antibiotics, and treatment cost [13]. For this reason, a granulocyte CSF can be used in appropriate cases, but it does not have known significant effect on morbidity.
Single-dose MTX therapy is used effectively in the treatment of EP. Although rare, it may be associated with myelosuppression and febrile neutropenia. Therefore, clinicians using MTX for this indication should be aware of this potential complication and act accordingly.
The authors declares no competing interests.
The author managed the patient, analyzed the patient data and read and agreed to the final manuscript.
Table 1: medical characteristics
Figure 1: A) generalized mild erythema; B) close-up view of erythema
- Marion LL, Meeks GR. Ectopic pregnancy: history, incidence, epidemiology, and risk factors. Clin Obstet Gynecol. 2012 Jun;55(2):376-86. PubMed | Google Scholar
- Anderson FWJ, Hogan JG, Ansbacher R. Sudden death: ectopic pregnancy mortality. Obstet Gynecol. 2004 Jun;103(6):1218-23. Google Scholar
- Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011 Feb 15;52(4):e56-93. PubMed | Google Scholar
- Lipscomb GH, Givens VM, Meyer NL, Bran D. Comparison of multidose and single-dose methotrexate protocols for the treatment of ectopic pregnancy. Am J Obstet Gynecol. 2005 Jun;192(6):1844-7; discussion 1847-8. PubMed | Google Scholar
- Baggott JE, Morgan SL, Vaughn WH. Differences in methotrexate and 7-hydroxymethotrexate inhibition of folate-dependent enzymes of purine nucleotide biosynthesis. Biochem J. 1994 Jun 15;300 (Pt 3)(Pt 3):627-9. PubMed | Google Scholar
- Puig L. Methotrexate: new therapeutic approaches. Actas Dermo-Sifiliográficas Engl Ed. Jul-Aug 2014;105(6):583-9. PubMed | Google Scholar
- Methotrexate - FDA. Accessed on 23 July, 2020.
- Jones KW, Patel SR. A family physician´s guide to monitoring Methotrexate. Am Fam Physician. 2000 Oct 1;62(7):1607-12, 1614. PubMed | Google Scholar
- Fisher MC, Cronstein BN. Metaanalysis of Methylenetetrahydrofolate Reductase (MTHFR) polymorphisms affecting methotrexate toxicity. J Rheumatol. 2009 Mar;36(3):539-45. PubMed | Google Scholar
- Faganel Kotnik B, Grabnar I, Bohanec Grabar P, Dolžan V, Jazbec J. Association of genetic polymorphism in the folate metabolic pathway with methotrexate pharmacokinetics and toxicity in childhood acute lymphoblastic leukaemia and malignant lymphoma. Eur J Clin Pharmacol. 2011 Oct;67(10):993-1006. PubMed | Google Scholar
- van Ede AE, Laan RF, Blom HJ, Huizinga TW, Haagsma CJ, Giesendorf BA et al. The C677T mutation in the methylenetetrahydrofolate reductase gene: A genetic risk factor for methotrexate-related elevation of liver enzymes in rheumatoid arthritis patients. 2001 Nov;44(11):2525-30. Google Scholar
- Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T et al. 2002 Guidelines for the Use of Antimicrobial Agents in Neutropenic Patients with Cancer. 2002 Mar 15;34(6):730-51. Google Scholar
- Ozer H, Armitage JO, Bennett CL, Crawford J, Demetri GD, Pizzo PA et al. 2000 update of recommendations for the use of hematopoietic colony-stimulating factors: evidence-based, clinical practice guidelines. J Clin Oncol. 2000 Oct 15;18(20):3558-85. PubMed | Google Scholar