Blood markers (lymphocyte percentages, neutrophils, CRP and ESR) can help in prioritizing rRT-PCR test for suspected COVID-19 patients in countries with limited health resources
Abd-Alhafeez Osman Ibnouf, Mohamed Hilal Khalil, Rayan Khalid, Elshibli Mohamed Elshibli, Osman Elsayed, Imad Fadl-Elmula
Corresponding author: Imad Fadl-Elmula, Department of Clinical Genetics, Alneelain Stem Cells Centre, Alneelain University, Khartoum, Sudan 
Received: 25 Jul 2020 - Accepted: 10 Oct 2020 - Published: 10 Dec 2020
Domain: Infectious diseases epidemiology
Keywords: COVID-19, blood markers, coronavirus
©Abd-Alhafeez Osman Ibnouf et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Abd-Alhafeez Osman Ibnouf et al. Blood markers (lymphocyte percentages, neutrophils, CRP and ESR) can help in prioritizing rRT-PCR test for suspected COVID-19 patients in countries with limited health resources. Pan African Medical Journal. 2020;37:331. [doi: 10.11604/pamj.2020.37.331.25180]
Available online at: https://www.panafrican-med-journal.com//content/article/37/331/full
Research 
Blood markers (lymphocyte percentages, neutrophils, CRP and ESR) can help in prioritizing rRT-PCR test for suspected COVID-19 patients in countries with limited health resources
Blood markers (lymphocyte percentages, neutrophils, CRP and ESR) can help in prioritizing rRT-PCR test for suspected COVID-19 patients in countries with limited health resources
Abd-Alhafeez Osman Ibnouf1, Mohamed Hilal Khalil2, .gif) Rayan Khalid3, Elshibli Mohamed Elshibli4, Osman Elsayed5, Imad Fadl-Elmula3,&
Rayan Khalid3, Elshibli Mohamed Elshibli4, Osman Elsayed5, Imad Fadl-Elmula3,&
&Corresponding author
Introduction: the outbreak of corona virus disease 2019 (COVID-19) started in China in December 2019 and spread causing more than 14 million cases all over the world on July 19th, 2020. Although, real-time reverse transcription polymerase chain reaction (rRT-PCR) test is the gold standard test, it needs a long time and requires specialized laboratories and highly trained personnel. All these difficulties forced many countries with reduced health resources to limit rRT-PCR tests to individuals with severe symptoms. Thus, routine blood marker that may help physicians to suspect COVID-19 and hence, prioritize patients for molecular diagnosis is badly needed.
Methods: fifty-six Sudanese COVID-19 patients admitted to Jabra hospital were included in this study. For all the patients we analyzed complete blood count (CBC), CBC, plasma levels of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), liver function tests (LFT) and renal function tests (RFT). Statistical analysis was done using SPSS program with a significance level of p≤0.05 and confidence limits (CLs) 95%. The difference between groups was tested using Mann-Whitney test was for quantitative variables while qualitative variables was tested using chi-square (Fisher exact) test.
Results: the result shows that, 35 out of the 56 patients (62.5%) were male and 21 (37.5%) were females with a median age of 60-year-old for both sexes. Lymphocytes % showed decrease to 9.2 (P-value=0.000) and significant increase in neutrophils to 83.05 (P-value=0.005), ESR to 65.54 (P-value=0.000) and CRP to 91.07 (P-value=0.000). The receiver operating characteristic curve (ROC)/area under the curve (AUC) ensured the expellant result of lymphocytes % as a predictor with 92% area under the curve, neutrophils were 90% and ESR 95.8%. The percent of detecting COVID-19 positive RT-PCR (98%) for suspected individuals using ROC showed best cutoff of ≤21.8 for lymphocytes %, ≥67.7 for neutrophils, ≥37.5 for ESR, ≥6.2 for CRP and ≥7.15 for WBCs.
Conclusion: the results also showed that, lymphocyte percentages, neutrophils, CRP and ESR may be used as markers for COVID-19 helping prioritizing individuals for rRT-PCR test.
A cluster of unexplained pneumonia cases were reported by the People´s Republic of China to the World Health Organization (WHO) on December 31st, 2019. By January 12th, 2020, China shared with the world the sequence of a novel virus (COVID-19) and later on the 13th of January 2020, Thailand confirms the first case of COVID-19 outside of China.
The etiology for this outbreak was a new corona virus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which was responsible for the corona virus disease 2019 (COVID-19) [1]. On July 23rd, 2020, the disease has spread to 213 countries worldwide with almost 15 million infected people and more than 618,017 deaths were reported to the WHO [2]. By March 13th, 2020, Sudan had confirmed the first case of COVID-19 infection using real time reverse transcriptase polymerase chain reaction (rRT-PCR), performed on respiratory samples of a patient that had returned from the UAE. By 24th July, a total of 11,237 patients were admitted and/or confirmed as COVID-19.
The rRT-PCR test remains the gold standard method for the etiological diagnosis of COVID-19 infection. Unfortunately, the maximum benefit use of rRT-PCR methods for diagnosis of COVID-19 was hindered in many countries, especially in the developing ones, by the limitation of molecular laboratories and well-trained personnel [3]. All these difficulties forced many countries with reduced health resources to limit the available rRT-PCR tests to individuals with pronounced respiratory syndrome symptoms [4]. This policy is reflected in under diagnoses of the disease due to reduction and/or delay in the number of tested patients for COVID-19 using RT-PCR, especially in those with non-classical COVID-19 presentation, which in turn led to increase community spread of the disease. This situation is seen in most African countries e.g. Sudan has only one testing center for COVID-19 with maximum capacity of 500 RT-PCR for COVID-19 test/day in Khartoum, which has population of 6 million inhabitants. Recent studies showed that some routine blood tests markers may help in prioritizing rRT-PCR for COVID-19 suspected patients in countries with limited health resources [5]. Thus, the aim of the present study is to identify the profile of routine blood test (markers) for COVID-19 patients to be used for prioritizing RT-PCR for COVID-19 suspected patients in countries with limited resource settings.
A total of 56 Sudanese COVID-19 patients (35 (62.5%) males and 21 (37.5%) females) admitted to Jabra Hospital, Khartoum, Sudan were included in this study. All the patients had respiratory symptoms and were tested positive for COVID-19 using rRT-PCR before admission to the isolation ward. For all patients, the complete blood count (CBC), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), total protein, albumin, total and direct bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), urea, creatinine and electrolytes were measured on admission of the patients.
Statistical analyses were done using statistical package for social sciences (SPSS) version 21 with a significance level of p≤0.05 and CLs 95%. The descriptive statistics (mean ±SD, median) was calculated to describe quantitative variables. The median was the best central measure because of the data abnormality, so the sign test was used instead of the parametric test and qualitative variables were described using frequency and percent. The differences between frequencies were tested by goodness of the fit test using chi-square or Fisher exact test when needed. The relationships between quantitative variables were tested by spearman correlation test, receiver operating characteristic curve and area under the curve (ROC/AUC) were used to obtain the true positive and false positive predictive values calculated using the best cutoff values.
The ethical clearance for conducting this study was obtained from the Ethical Committee Board of Assafa Academy. Patients were not contacted directly; data and laboratory results were obtained from hospital archive and kept anonymous at all stages of the study.
Of the 56 patients, 35/62.5% were male and 21/37.5% were females, all were a Sudanese mixture of Nilo-Saharan and Afro-Asiatic ethnic origin and all were COVID-19 positive using rRT-PCR at the time of admission. Their age ranging between 10 - 82-year-old and the median age was 60-year-old. The males were significantly older (median 62) than the females (median 50) using Mann-Whitney test with P-value=0.003.
The results showed that the plasma median levels of lymphocytes, neutrophils, CRP, ESR, urea, Na+ and K+ were altered being higher or lower than normal (Table 1). For the red blood cells (RBCs), white blood cells (WBCs), platelets (PLTs), Hemoglobin (Hb), total protein, albumin, liver enzymes and creatinine the median values were within normal level (Table 1).
The analysis showed significant frequency distribution amongst patients groups with normal, high or low values using chi-square test for lymphocytes (P=0.000), neutrophils (P=0.000), PLT (P=0.000), CRP (P=0.000), ESR (P=0.000), total protein (0.001), albumin (P=0.000), direct bilirubin (P=0.000), urea (P=0.003), creatinine (P=0.001), Na+ (P=0.000), and K (P=0.001) (Table 2). For the remaining variables (RBCs, Hb, WBCs, total bilirubin, AST, ALT, ALP) no statistical significance was seen amongst patients´ groups of normal, high or low values (Table 2).
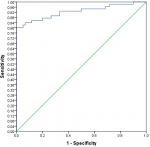
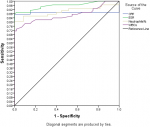
Analysis using Pearson correlation test showed an inverse relationship between lymphocytes and neutrophils (P=0.000) and between lymphocytes and WBCs (P=0.000). The receiver operating characteristic curve (ROC)/area under the curve (AUC) ensure the expellant result of lymphocytes percentage as a predictor with 92% AUC, neutrophils were 90% AUC and ESR 95.8% AUC. The good result recorded for CRP was 89% AUC and WBCs were 86.8% AUC. The percentage of detecting COVID-19 positive patient using RT-PCR is (98%), for suspected individuals using ROC and the best cutoff for lymphocytes percentage (≤21.8), neutrophils (≥67.7), ESR (≥37.5), CRP (≥6.2) and WBCs (≥7.15). The true positive and false positive presented in Table 3, Figure 1 and Figure 2.
The rRT-PCR test remains the gold standard method for the etiological diagnosis of SARS-CoV-2 infection. In addition to the demands and limitations of this technique even for developed countries, there is also a need for certified laboratories with expensive equipment and highly trained personnel [6]. The challenges are even more for developing countries and those with limited health resources due to shortage of specialized laboratories and/or increased reagents cost. These limitations were translated in very limited numbers of qualified molecular laboratories capable of testing, leading to an extremely long waiting list of COVID-19 suspected patients. Furthermore, the main objective of the present study is to find sensitive and predictive blood markers so as to help in prioritizing those with high vulnerability and susceptibility of being infected.
Although, many researches focus on the correlation of blood markers and the severity of the disease and/or its outcome, not many studies investigate the role of blood markers in prioritizing rRT-PCR for COVID-19 suspected patients in countries with limited health resources. If such markers turn out to be of high sensitivity and specificity in predicting patients with COVID-19 infection, it will reduce the long waiting list of molecular testing, improving the morbidity and reducing the mortality with COVID-19 in developing countries. In our study, 4 markers (lymphocytes, neutrophils, CRP and ESR) were potentially important in predicting who will show positive PCR in COVID-19 (Table 3). Most of these markers were reported in other studies showing good correlation between the blood marker and the disease progression and outcome [7]. In our study, the results showed WBCs normal median range in most of COVID-19 patients on their admission day (Table 2). This result of WBCs was in complete accordance with what has been reported in the literature [8]. However, 78.6% of patients showed low lymphocyte counts (lymphopenia) and statically significant frequency distribution with a P-value=0.000. Comparing our result with the previous studies, our data showed even stronger correlation between the lymphopenia and COVID-19 infection with a predictor value of 92% (sensitivity and specificity) [8,9].
Lymphopenia was also observed in the previous two outbreaks that were caused by coronaviruses; sever acute respiratory syndrome (SARS) in 2003 and Middle East Respiratory Syndrome (MERS) in 2012. The pathogenesis of lymphopenia as recent studies revealed, include direct viral infection, immune mediated lymphocytes destruction and cytokine-mediated altered lymphocyte trafficking and sequestration [10,11]. The decline in lymphocytes count is used in evaluation of severity and outcome of COVID- 19 infection [12].
In addition to lymphocytes %, an increase in 3 other blood markers (neutrophils, CRP, ESR) showed high prediction for COVID-19 infection. Similar data was reported for CRP, ESR and neutrophils [8,13]. We observed no association between Hb, platelets and the disease (Table 2); this was in contrast to the study conducted by Chen et al. [14], which showed a significant association between low Hb, thrombocytopenia and COVID-19 patients. However, we acknowledge that our study suffers from a few limitations like the relatively limited number of patients and the absence of a controlled population, clinical signs which can help in discriminating between positive and negative COVID-19 patients.
According to the present study, physicians in countries with limited testing resources may need to include blood markers (lymphocytes, neutrophils, CRP and ESR) to prioritize the patients with a high suspicion of having COVID-19 for rRT-PCR test based on these markers. If done it may reduce the false-negative number of COVID-19 patients and improve the clinical course of the disease by improving morbidity and reducing the mortality in those patients.
What is known about this topic
- The gold standard diagnostic test for COVID-19 is identification of virus RNA using rRT-PCR technique;
- The molecular diagnosis required specialized molecular virology labs and highly trained personnel; both are limited in developing countries with low health resources.
What this study adds
- The present study provides blood markers (lymphocytes %, neutrophils, CRP and ESR) that can be used to increase the predictively for the diagnosis of COVID-19 patients, which may help physicians to prioritize rRT-PCR suspected patients who really need to do confirmatory molecular test;
- The blood markers suggested by this study may help countries with limited health resources to maximize the benefit use of their limited diagnostic resources so as to reduce rRT-PCR false negative results; that may lead to decrease morbidity by early detection and isolation of confirmed COVID-19 positive cases.
The authors declare no competing interests.
All the authors have read and agreed to the final manuscript.
Table 1: descriptive statistics of blood markers among Sudanese COVID-19 patients
Table 2: the frequency distribution of blood markers among Sudanese COVID-19 patients
Table 3: predictive values from receiver operating characteristic curve and area under the curve (ROC/AUC)
Figure 1: the receiver operating characteristic curve and area under the curve (ROC/AUC) of lymphocytes percentage
Figure 2: the receiver operating characteristic curve and area under the curve (ROC/AUC) of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), neutrophils and white blood cells (WBCs)
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536-544. PubMed | Google Scholar
- Islam MT, Talukder AK, Siddiqui MN, Islam T. Tackling the pandemic COVID-19: the Bangladesh perspective. Preprint. 2020. Google Scholar
- Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med CCLM. 2020;58(7):1131-1134. PubMed | Google Scholar
- Cohen J, Kupferschmidt K. Countries test tactics in war against COVID-19. American Association for the Advancement of Science. 2020;367(6484):1287-1288. PubMed | Google Scholar
- Ferrari D, Motta A, Strollo M, Banfi G, Locatelli M. Routine blood tests as a potential diagnostic tool for COVID-19. Clin Chem Lab Med CCLM. 2020;58(7):1095-1099. PubMed | Google Scholar
- Tiede I, Fritz G, Strand S, Poppe D, Dvorsky R, Strand D et al. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest. 2003;111(8):1133-1145. PubMed | Google Scholar
- Velavan TP, Meyer CG. Mild versus severe COVID-19: laboratory markers. Int J Infect Dis. 2020;95:304-307. PubMed | Google Scholar
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020;395(10223):497-506. PubMed | Google Scholar
- Xu X-W, Wu X-X, Jiang X-G, Xu K-J, Ying L-J, Ma C-L et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. bmj. 2020;368:m606. PubMed | Google Scholar
- Ko J-H, Park GE, Lee JY, Lee JY, Cho SY, Ha YE et al. Predictive factors for pneumonia development and progression to respiratory failure in MERS-CoV infected patients. J Infect. 2016;73(5):468-475. PubMed | Google Scholar
- Azkur AK, Akdis M, Azkur D, Sokolowska M, van de Veen W, Brüggen M-C et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75(7):1564-1581. PubMed | Google Scholar
- Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846-848. PubMed | Google Scholar
- Ling W. C-reactive protein levels in the early stage of COVID-19. Med Mal Infect. 2020;50(4):332-334. PubMed | Google Scholar
- Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382(17):e38. PubMed | Google Scholar