Clinical and histological patterns and treatment of pyoderma gangrenosum
Radia Chakiri, Hanane Baybay, Asmae El Hatimi, Salim Gallouj, Taoufiq Harmouch, Fatima Zohra Mernissi
Corresponding author: Radia Chakiri, Department of Dermatology, University Hospital Hassan II, Fez, Morocco 
Received: 24 Mar 2017 - Accepted: 08 Jul 2017 - Published: 02 Jun 2020
Domain: Dermatology
Keywords: Pyoderma gangrenosum, neutrophilic dermatosis, systemic disease
©Radia Chakiri et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Radia Chakiri et al. Clinical and histological patterns and treatment of pyoderma gangrenosum. Pan African Medical Journal. 2020;36:59. [doi: 10.11604/pamj.2020.36.59.12329]
Available online at: https://www.panafrican-med-journal.com//content/article/36/59/full
Clinical and histological patterns and treatment of pyoderma gangrenosum
Radia Chakiri1,&, Hanane Baybay1, Asmae El Hatimi1, Salim Gallouj1, Taoufiq Harmouch2, Fatima Zohra Mernissi1
1Department of Dermatology, University Hospital Hassan II, Fez, Morocco, 2Department of Anatomopathology, University Hospital Hassan II, Fez, Morocco
&Corresponding author
Radia Chakiri, Department of Dermatology, University Hospital Hassan II, Fez, Morocco
Pyoderma gangrenosum (PG) is a rare inflammatory neutrophilic dermatosis for which accurate epidemiological data are limited and therapy remains a challenge. The primary study´s aim was to examine all cases of PG observed in our department over a 6-year period in order to describe the relevant characteristics and outcome under therapy. Fourteen patients were included (5 women, 9 men). The average age of our patients was 40,15 years. The classical, ulcerative form was found in 10 cases (71.42%), the pustular form in 4 cases (27.57%) and PG was multifocal in 4 cases. The PG was located preferentially to the lower limbs. Histological examination was realized in all patients and objectified inflammatory infiltrate composed of polymorphonuclear neutrophils in all cases with vasculitis in 4 cases. Six patients (42.85%) had associated disease at diagnosis of PG, including inflammatory bowel disease in two cases (14.28%), a blood disease in 2 cases (14.28%), lymph node tuberculosis and inflammatory arthritis in 1 case (7%). The most frequent first-line treatments were oral corticosteroids (7 cases) and other treatments used were colchicine in 2 cases, topical corticosteroids in 3 cases with good clinical evolution. Our study confirms that PG is a rare disease, associated in almost half of cases with systemic disease already present at diagnosis; in our Moroccan background, it is most often inflammatory bowel disease, hematological or solid cancer and tuberculosis.
Pyoderma gangrenosum (PG), first described by Brocq in 1916 and named by Brunsting et al. in 1930, is a rare non infectious neutrophilic dermatosis that typically presents as destructive cutaneous ulcerations that usually appear on the legs [1, 2]. PG can occur at any age; however, it generally presents in the second to fifth decades of life. A century after its first description, we have found out that it was not related to an infection despite its original definition, but we still do not fully understand the pathophysiology of PG. Currently, the loss of innate immune regulation and altered neutrophil chemotaxis are believed to be involved to some extent and considering its association with other auto-inflammatory diseases such as Crohn disease and Behcet´s disease, PG is now included within the spectrum of systemic auto inflammatory diseases [1,3]. The hallmark of the disease is painful ulcerations that can affect any area of the body but are most commonly found in the lower legs.
In the classical type, skin lesions arise suddenly as painful, tender, erythematous papules, plaques, nodules or pustules that rapidly progress to expanding ulcers with characteristic violaceous undermined edges and a necrotic base. Healing of these ulcers usually results in characteristic atrophic cribriform scars [1, 4, 5]. A diagnostic criterion has been also defined [6]; however, it has not been uniformly accepted according to a study [7]. Thus, diagnosis is solely based on clinical findings and exclusion of other ulcerating skin diseases [1, 5]. PG occurs in association with an underlying disease such as inflammatory bowel disease (IBD), inflammatory arthritis, hematological disorders and solid malignancies in 50% to 70% of the cases [4].
Thus, investigations are also necessary to determine whether there is a treatable systemic, associated disorder. Remaining cases are rather idiopathic or related to other factors such as trauma or surgery [5,7]. Recent studies of PG associated genetic syndromes may provide insight into the pathogenesis of PG and may help develop specific therapies against new targets [3,5]. PG is a relatively rare disease that makes it difficult to obtain results from randomized controlled studies and treatments are traditionally individualized according to patient compliance and associating systemic diseases. Thus, there is no standard treatment protocol for PG patients [1, 4, 7]. The primary aim of our study was to examine all cases of PG observed in our department over a 6-year period in order to describe the relevant characteristics and outcome under therapy.
We present a monocentric prospective, observational study included PG patients who were treated between 2009 and 2015 in the Department of Dermatology, University Hospital Hassan II, Fez, Morocco. Cases were identified by screening for patients with a discharge diagnosis of PG that was based on clinical findings and histopathological features consistent with PG together with investigations to exclude other causes including infections, tumors or vasculitis. Age, gender, clinical and histological findings, demographics, comorbidities, therapeutic modalities and outcome were recorded.
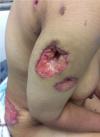
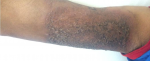
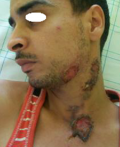
Clinical and histopathological characteristics: the clinical characteristics of the patients at diagnosis of PG are summarized in Table 1. For the 14 patients included (5 women, 9 men; sex ratio H / F = 0.4), the average age at diagnosis was 40.15 years (range: 11-70 years). The classical, ulcerative form was found in 10 cases (71.42%) (Figure 1) and the pustular form in 4 cases (27.57%) (Figure 2). The PG was located preferentially to the lower limbs in 10 cases, trunk, upper limb and neck (Figure 3) in one case each. Histological examination showed a dense infiltrate of neutrophil in all patients, this infiltrate was associated with vasculitis in four cases and lymphoplasmacytic infiltrate in five cases.
Associated diseases: six patients (42,85%) had associated disease at diagnosis of PG, including inflammatory bowel disease in 2 cases (14.28%), a blood disease in 2 cases (14.28%), lymph node tuberculosis and inflammatory arthritis in one case each (7%). In our series one patient was pregnant at 6 month.
Treatments and follow-up: the patients included in the study were followed for varying periods ranging from 3 months to 4 years. Treatment with oral steroids (Prednisone: 0.5-1mg / kg / day) was prescribed first line in 9 cases (64.28%), alone or combined with topical treatment. Topical corticosteroids were used in first line in three cases (21.42%). The remaining two cases were treated with colchicine 1 mg/day.
Pyoderma gangrenosum may occur at any age but typically affects those between 20 and 50 years (51.8%), as described by our study and in previous studies [8-10]. In our series we noted a male predominance; however, in most series reported in the literature pyoderma gangrenosum affects women more than men. The clinical characteristics of PG in our study are comparable to published data, with a predominance of the classical ulcerative form. Other clinical variant, bullous, pustular, vegetative and peristomal types are possible but rare. Lesions can appear on any part of the body, but ulcers of the classical subtype favor the lower extremities in up to 85.7% of the patients [11-13], as was the case for 71.42% of our patients. The reason for this specific location is not known [13]. Binus et al. [14] found that almost one third of the patients had comorbid conditions such as diabetes and/or peripheral vascular disease and raised the question that these 2 diseases might play a role in and contribute to the development of PG and worsen local healing processes, especially on the lower leg.
The diagnosis of PG relies on clinical signs first and is supported by biopsy for histopathology. Knowledge of the patient's history for possible underlying disease and specific investigations based on that background are necessary. Therefore, diagnosis is made by exclusion of other possible disorders. No laboratory parameter for PG is available. The histopathology of PG is no specific and changes with the stage of lesion. The initial lesions show a deep suppurative folliculitis with dense neutrophilic infiltrate. In about 40% of cases, leukocytoclastic vasculitis is present as was in four of our cases. PG with (necrotizing) granulomatous inflammation has been described [15-17]. An associated disease, similar to those observed in other neutrophilic dermatosis, was found in 42.85% of our patients, which is consistent with the literature, where the frequency of pathological associations is between 33 and 84% [1, 18-20].
IBD was identified in 14.28% of cases (20% to 30% in the literature), a blood disease in 14,28% of cases (15% to 25% in the literature), inflammatory arthritis in 7% of cases (20% to 30% in the literature) and ganglionic tuberculosis in 7% of our cases (extremely rare association in the literature). However, thyroid diseases (including cancer of the thyroid) were very rare [18]. Because the etiology of the disease is not being understood, there is no specific and uniformly effective therapy for PG. The aim of the treatment is to reduce pain and to promote wound healing by reducing inflammation with anti-inflammatory and immunosuppressive agents, thereby improving the quality of life of PG patients.
Thus for extended and active lesions, treatments which have shown efficacy in several case series are oral corticosteroid therapy with prednisone doses ranging from 40 to 120 mg per day in adults and cyclosporine (average dose of 5 to 7 mg / kg / day) [21-23]. Our study had some limitations related to the assessment of a small number of PG patients and patient recruitment through the only dermatology department which was originally a selection bias. Thus, the results of this study need to be confirmed by multicenter studies with large number of patients and especially by involving other specialists for patient recruitment.
Our study confirms that the PG is a rare disease, associated in almost half of cases with systemic disease already present at diagnosis; it is most often IBD, blood disorders, solid cancers and tuberculosis in our Moroccan background.
What is known about this topic
- PG a rare inflammatory neutrophilic dermatosis;
- Association with systemic diseases.
What this study adds
- The first study of PG in Morocco;
- Tuberculosis association with PG in our background.
The authors declare no competing interests.
All the authors have read and agreed to the final manuscript.
Table 1: clinical and histopathological characteristics of patients
Figure 1: disseminated ulcerative pyoderma gangrenosum
Figure 2: pustular pyoderma gangrenosum
Figure 3: ulcerative pyoderma gangrenosum of the neck
- Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13(3):191-211. PubMed | Google Scholar
- Gameiro A, Pereira N, Cardoso JC, Goncalo M. Pyoderma gangrenosum: challenges and solutions. Clin Cosmet Investig Dermatol. 2015;8:285-293. PubMed | Google Scholar
- Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol. 2015;73(4):691-698. PubMed | Google Scholar
- Ruocco E, Sangiuliano S, Gravina AG, Miranda A, Nicoletti G. Pyoderma gangrenosum: an updated review. J Eur Acad Dermatol Venereol. 2009;23(9):1008-1017. PubMed | Google Scholar
- Patel F, Fitzmaurice S, Duong C, He Y, Fergus J, Raychaudhuri SP et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95(5):525-531. PubMed | Google Scholar
- Su WP, Davis MD, Weenig RH, Powell FC, Perry HO. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43(11):790-800. PubMed | Google Scholar
- Wollina U. Pyoderma gangrenosum: a systemic disease. Clin Dermatol. 2015;33(5):527-530. PubMed | Google Scholar
- Powell FC, Schroeter AL, Su WP, Perry HO. Pyoderma gangrenosum: a review of 86 patients. Q J Med. 1985;55(217):173-186. PubMed | Google Scholar
- Ye MJ, Ye JM. Pyoderma gangrenosum: a review of clinical features and outcomes of 23 cases requiring inpatient management. Dermatol Res Pract. 2014 Oct 8;2014:461467. PubMed | Google Scholar
- Adisen E, Erduran F, Gürer MA. Pyoderma gangrenosum: a report of 27 patients. Int J Low Extrem Wounds. Juin 2016;15(2):148-54. PubMed | Google Scholar
- Von den Driesch P. Pyoderma gangrenosum: a report of 44 cases with follow-up. Br J Dermatol. 1997;137(6):1000-1005. PubMed | Google Scholar
- Mlika RB, Riahi I, Fenniche S, Mokni M, Dhaoui MR, Dess N et al. Pyoderma gangrenosum: a report of 21 cases. Int J Dermatol. 2002;41(2):65-68. PubMed | Google Scholar
- Pereira N, Brites MM, Goncalo M, Tellechea O, Figueiredo A. Pyoderma gangrenosum a review of 24 cases observed over 10 years. Int J Dermatol. 2013;52(8):938-945. PubMed | Google Scholar
- Binus AM, Qureshi AA, Li VW, Winterfield LS. Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol. 2011;165(6):1244-1250. PubMed | Google Scholar
- Crowson AN, Mihm MC Jr, Magro C. Pyoderma gangrenosum: a review. J Cutan Pathol. 2003;30(2):97-107. PubMed | Google Scholar
- Su WP, Schroeter AL, Perry HO, Powell FC. Histopathologic and immunopathologic study of pyoderma gangrenosum. J Cutan Pathol. 1986;13(5):323-330. PubMed | Google Scholar
- Park HJ, Kim YC, Cinn YW, Yoon TY. Granulomatous pyoderma gangrenosum: two unsual cases showing necrotizing granulomatous inflammation. Clin Exp Dermatol. 2000;25(8):617-620. PubMed | Google Scholar
- Al Ghazal P, Herberger K, Shaller G, Strölin A, Hoff NP, Goerge T et al. Associated factors and comorbidities in patients with pyoderma gangrenosum in Germany: a retrospective multicentric analysis in 259 patients. Orphanet J Rare Dis. 2013;8:136. PubMed | Google Scholar
- Langan SM, Groves RW, Card TR, Gulliford MC. Incidence, mortality and disease associations of pyoderma gangrenosum in the United Kingdom: a retrospective cohort study. J Invest Dermatol. 2012;132(9):2166-70. PubMed | Google Scholar
- Duarte AF, Nogueira A, Lisboa C, Azevedo F. Pyoderma gangrenosum-clinical, laboratory and therapeutic approaches: review of 28 cases. Dermatol Online J. 2009;15(7):3. PubMed | Google Scholar
- Powell F, Su W, Perry H. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34(3):395-409. PubMed | Google Scholar
- Teagle A, Hargest R. Management of pyoderma gangrenosum. JR Soc Med. 2014;107(6):228-36. PubMed | Google Scholar
- Dabade TS, Davis MD. Diagnosis and treatment of the neutrophilic dermatoses (pyoderma gangrenosum, Sweet´s syndrome). Dermatol Ther. 2011;24(2):273-84. PubMed | Google Scholar