Seroprevalence of brucellosis and associated factors among livestock slaughtered in Oko-Oba abattoir, Lagos State, southwestern Nigeria
Kenneth Onyebuchi Ukwueze, Olayinka Olabisi Ishola, Magbagbeola David Dairo, Emmanuel Jolaoluwa Awosanya, Simeon Idowu Cadmus
Corresponding author: Olayinka Olabisi Ishola, Department of Veterinary Public Health and Preventive Medicine, Faculty of Veterinary Medicine, University of Ibadan, Ibadan, Nigeria 
Received: 25 Nov 2019 - Accepted: 19 Feb 2020 - Published: 02 Jun 2020
Domain: Bacteriology,Epidemiology,Microbiology
Keywords: Seroprevalence, Brucella infection, Abattoir, Lagos State, Livestock
©Kenneth Onyebuchi Ukwueze et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Kenneth Onyebuchi Ukwueze et al. Seroprevalence of brucellosis and associated factors among livestock slaughtered in Oko-Oba abattoir, Lagos State, southwestern Nigeria. Pan African Medical Journal. 2020;36:53. [doi: 10.11604/pamj.2020.36.53.21094]
Available online at: https://www.panafrican-med-journal.com//content/article/36/53/full
Research 
Seroprevalence of brucellosis and associated factors among livestock slaughtered in Oko-Oba abattoir, Lagos State, southwestern Nigeria
Seroprevalence of brucellosis and associated factors among livestock slaughtered in Oko-Oba abattoir, Lagos State, southwestern Nigeria
Kenneth Onyebuchi Ukwueze1, Olayinka Olabisi Ishola2,&, Magbagbeola David Dairo3, Emmanuel Jolaoluwa Awosanya2, Simeon Idowu Cadmus2
1Nigeria Field Epidemiology and Laboratory Training Programme, Abudja, Nigeria, 2Department of Veterinary Public Health and Preventive Medicine, Faculty of Veterinary Medicine, University of Ibadan, Ibadan, Nigeria, 3Department of Medical Statistics and Epidemiology, Faculty of Public Health , College of Medicine, University of Ibadan, Ibadan, Nigeria
&Corresponding author
Olayinka Olabisi Ishola, Department of Veterinary Public Health and Preventive Medicine, Faculty of Veterinary Medicine, University of Ibadan, Ibadan, Nigeria
Introduction: Brucella infection, a neglected tropical zoonosis, poses public health threat to abattoir workers in developing countries including Nigeria. Oko-Oba abattoir is one of the largest abattoirs in the country that collects livestock from different parts of the country. This study determined the prevalence and factors associated with seropositivity of brucellosis among livestock slaughtered at Oko-Oba abattoir.
Methods: a cross-sectional study was conducted from January to May 2018. A total of 473 serum samples were collected from livestock at the abattoir and tested for antibodies to Brucella species using the Rose Bengal Plate Test (RBPT) and indirect Enzyme Linked Immunosorbent Assay (iELISA). Data were analyzed using descriptive statistics and chi square test (p < 0.05).
Results: overall seroprevalence values were 15.3% (RBPT) and 16.3% (iELISA) among the livestock slaughtered at the Oko-Oba abattoir. Seroprevalence of 17.2% (RBPT) and 15.8% (iELISA) in cattle; 15.1% (RBPT) and 14.5% (iELISA) in goat; and 8.3% (RBPT) and 23.3% (iELISA) in sheep were obtained. Higher seroprevalence were recorded among females in cattle (18.8% iELISA) and sheep (23.1% iELISA) while male goats had average value higher (14.7% iELISA) than the female (p > 0.05).
Conclusion: presence of Brucella infection among slaughtered livestock was confirmed at Oko-Oba abattoir, Lagos State, Nigeria and poses a threat to abattoir workers and public health. Control of the disease in livestock and use of personal protective gear is recommended.
Brucellosis occurs worldwide but is better controlled in developed countries by routine screening of domestic animals and vaccination. The disease is a major neglected zoonosis of developing countries including Nigeria [1]. However, the prevalence varies in time and space among different livestock [2]. Brucellosis is endemic in sub-Saharan Africa, with significant effects on economic and social conditions of people in this region [3,4]. The incidence is influenced by management factors, herd size, population density, type of animal breed and biological features such as herd immunity [5, 6]. McDermott and Arimi [7] made a summary of data in cattle prior to 2001 which varied from 7.5% to 40% for pastoralists in arid and semiarid areas, 0.3-25.4% for cash/subsistence crops with livestock in sub-humid areas, 1.5-16.2% for crop-livestock in tropical highlands and 2.4-45.0% for crop with small-scale livestock production in humid areas. Studies reported seroprevalence of 2.9% with iELISA in argentine Creole sheep, 12.0% in Nepal and 4.1% to 6% in Costa Rica among cattle, respectively [8-10]. However in Ethiopia, 1.6% was reported in pastoral goats [11]. It is evidenced that the distribution and seroprevalence of brucellosis differs with species and location.
In Nigeria, several serological investigations have shown that Brucella infection is endemic in livestock population. In cattle, prevalence of 8.6% in Lagos state [12]; and 37.0% in three northern states (Kaduna, Kano and Adamawa) [13] have been reported. Also, another study in north-central Nigeria reported prevalence of 16.1% in cattle and most recent abattoir study in Ibadan, south-west Nigeria recorded a prevalence of 7.8% among cattle slaughtered at the abattoir [14]. Most of the investigation were done in cattle, although few documented evidence shows that the disease also exist in small ruminants, with prevalence of 0.86%, 14.0% and 25.80% reported in south-west, north-east and north-central Nigeria, respectively [15, 16]. Ogugua et al.[17] reported a prevalence of 2.83% among goats across four states in Nigeria (Borno, Oyo, Benue and Sokoto). However, the rates of Brucella infections recorded among cattle in these states is useful because it is a common practice for cattle and small ruminants to share common grazing and watering points in the north. This variation in prevalence may be due to geography, animal source, sample size, sampling technique and interpretation of tests [17]. Most importantly, a large number of animals in slaughter houses are culled by farmers for unproductiveness which could be indicative of diseases. The source and location of animals serve as potential risk factors in the epidemiology of brucellosis in animals. The widespread incidence of brucellosis could transmit to increase in human cases [4], especially among individuals at risk. There was therefore, need to undertake this evidence-based and multidisciplinary study of Brucella infection in different livestock slaughtered at an abattoir to evaluate the potential impact of Brucella infection and to inform effective control strategies. Hence, this study aimed at determining the seroprevalence of Brucella infection in livestock slaughtered at Oko-Oba abattoir and identifying possible risk factors.
Study design: we conducted a cross sectional study to determine the prevalence and factors responsible for seropositivity of Brucella infection in sera of animals slaughtered at Oko-Oba abattoir, Lagos between January and May 2018.
Study setting: the study was at an abattoir facility in Oko-oba, Agege, Lagos State, Nigeria. Lagos State is bounded in the north and east by Ogun State, in the south by Atlantic Ocean and in the west by the Republic of Benin. The international boundary with the Republic of Benin is a unique opportunity for cross-border trading. It occupies a land area of 3,577 square kilometers [18]. The projected population of Lagos State from Nigeria´s 2006 National census is estimated to be 15 million [18]. About 75% of the population lives in urban areas. Oko-Oba abattoir ranked the largest abattoir in Nigeria and biggest in West Africa [19]. About 3000 livestock are slaughtered daily. It has an automated slaughtering line, rendering units and waste treatment plant [20]. The abattoir accounts for 50% of meat consumed in Lagos State.
Study subjects: the study population was made up of all livestock that were slaughtered at the Oko-Oba abattoir. Cattle, goats and sheep at the abattoir at the time of sample collection were included in the study while all cattle, goats and sheep at the abattoir whose owner did not consent at the time of sampling were excluded.
Sample size consideration: the sample size was determined based on prevalence of 8.6% [12], 2.83% [17] and 3.6% [21] Brucella infection reported for cattle, goats and sheep, respectively. The formular
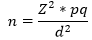 .
.
for sample size calculation in a cross sectional study was used. The reliability coefficient (Z) is 1.96 at 95% confidence level. The margin of error (d) of 4% was used for both cattle and sheep; while 2.5% was used for goats for good precision. All sample size calculations were adjusted for 10% non-response rate; this gave a sample size (n) of 215 cattle, 178 goats and 91 sheep. However, 221 cattle, 192 goats and 60 sheep (due to low number of sheep slaughtered throughout the study period) were used for the study.
Data collection and collection of serum samples from the livestock: three milliliters (ml) of venous blood was collected under strict hygienic conditions using sterile syringes and needles from cattle, goats and sheep just before slaughter into labeled sterile 5ml plain serum tubes and kept in slanted position on ice for serum formation. Serum samples were transported to the laboratory on ice for further processing. Each serum sample was labeled with a code that corresponds to the study site and subject identification. Intrinsic factors such as sex, age, body score and breed were recorded for each livestock species accordingly.
Serological testing: the Rose Bengal plate test (RBPT) consisting of standardized Brucella abortus and Brucella melitensis antigens sourced from the Animal Health and Veterinary Laboratories Agency, Weybridge U.K. was used for the Brucella test. Accurately, equal volumes (30μl) of antigen and test serum were mixed thoroughly on a plate using a spatula and the plate was rocked for 4 minutes. The appearance of agglutination within 2 minutes was scored 2+ (++), while the agglutination after 2 minutes was scored 1+ (+). The absence of agglutination after 4 minutes was scored negative (-). However, all cattle and sheep, and 179 of goat samples were further subjected to indirect enzyme linked immuno-sorbent assay (iELISA), using kit which was sourced from IDvet Innovative Diagnostics, Grabels, France; a multi-specie indirect ELISA diagnostic kit designed for detection of antibodies directed against Brucella abortus (bovine), B. melitensis (ovine and caprine) and B. suis (swine) in serum. The test was carried out according to the manufacturer´s procedure. The outcome was measured by ELISA reader and read as Optical Density (O.D.) values at 450nm. We defined a seropositive animal as any serum on screening for the presence of antibodies which tested positive to either RBPT or has sample / positive O.D. percentage of greater than or equal to 110% result by the iELISA. Seronegative animal is any animal whose serum was collected at the same time with the seropositive animal and on screening tested negative to RBPT or has a sample / positive O.D. of less than 110% result by the iELISA.
Data analysis: the age of the animal was determined using estimation of age by teeth appearance [22]. The body conditions were assessed by clinicians through examination and grading based on automatic grading of livestock [23]. Data were analyzed using Epi-Info version 7.2 software. Demographic characteristics were measured as the independent variables while the outcome of the serological test was measured as the dependent variables. We determined frequencies, proportions, and odds ratios. Chi square test was used to test level of significance in the values of the odds ratios at p < 0.05.
Ethical considerations: ethical approval for this study was obtained from the Lagos State Ministry of Agriculture. Permission was also obtained from the management of the abattoir where the study was conducted. Voluntary informed consent was explained in writing and obtained from the owners of each eligible animal before sample collection.
Of the 221 cattle sampled; most 154 (69.7%) were cows; and 81 (36.7%) were Rahaji breed, followed by Adamawa Gudali 37 (16.7%) and Ambala having the least 13 (5.9%). Most 218 (98.6%) of the cattle were adult, while 139 (62.9%) had good body condition. Of the 192 goats, the most breed 126 (65.6%) was Red Sokoto and 190 (98.9%) had good body condition. Of the 60 sheep, the most common breed 45 (75´0%) was Uda, and 51 (98.3%) were adults (Table 1). Overall seroprevalence of Brucella infection at the abattoir by Rose Bengal Plate Test (RBPT) was 15.3% (72 of 473); while by indirect Enzyme Linked Immuno-sorbent Assay (iELISA) was 16.3% (75 of 460). The seroprevalence of Brucella infection by RBPT in cattle, goats and sheep were 17.2%, 15.1% and 8.3%, respectively. By iELISA, Brucella infection seroprevalence of 15.8%, 14.5 % and 23.3% were obtained for cattle, goats and sheep, respectively (Table 2). There was no statistical difference (p > 0.05) in the Brucella infection seroprevalence among the species (Table 3). For breed-specific seroprevalence; the highest (33.3%) was among Cross breed in cattle by RBPT; and 30.8% among Ambala breed by iELISA, In goats, the highest breed specific seroprevalence of 16.7% was among Red Sokoto by RBPT; and among Sahel breed (25.0%) by iELISA. In sheep, the Uda breed has the highest seroprevalence both by RBPT (8.8%) and iELISA (24.4%); the West African Dwarf (WAD) breed was negative for both tests (Table 2). None of the intrinsic factors such as sex, age, body score and breed in all the livestock species-cattle, goats and sheep was significantly associated with Brucella infection both by RBPT (Table 3) and iELISA (Table 4). However, in cattle, the Ambala breed was eight times more likely (Odds ratio: 7.7; 95% confidence interval: 1.2-49.2) to be Brucella positive by iELISA compared to the Adamawa Gudali (Table 4).
The study confirmed that Brucella infection is present in cattle, goats and sheep slaughtered for consumption at Oko-Oba abattoir, Lagos and that seroprevalence varied across species, breed, sex and age of animals. In this study, two serological tests were adopted to confirm the seroprevalence of the livestock using serum samples. This method was also adopted by Kashiwazaki et al [24] who demonstrated that combination of RBPT for screening of infected herds and the iELISA for identifying infected individuals was considered to be a quite appropriate and effective diagnostic tool for large scale serological survey of brucellosis. The RBPT is susceptible to cross reaction with other gram negative bacteria such as Yersinia enterocolitica O:9, Eschrichia coli O:157; and some Salmonella species, which could lead to false positive results [25]. Nonetheless, these two methods are suitable for the detection of Brucella antibodies in cattle and small ruminants. The study revealed an overall prevalence of 15.2% and 16.3% with the RBPT and iELISA, respectively among the livestock slaughtered at the abattoir. This was much higher than 2.6% reported among livestock in Niger [21] and 5.8% recorded by Assenga et al in Tanzania [26]. A seroprevalence of 17.2% and 15.8% was observed among slaughtered cattle based on RBPT and iELISA, respectively. This is higher than the prevalence of 8.6% earlier reported among cattle in Lagos by Cadmus [12] and much higher than 4.9% reported by Ogugua et al [27] in South Western Nigeria and 5.45% recorded by Bwala et al [19] in Ibadan municipal abattoir Nigeria. Although, it was lower than 36.6% recorded among cattle in three northern states [13]. However the seroprevalence obtained in this study is much higher compared to other reports from developing countries which reported 2.21% in South Africa [28], and 2.90% in Ethiopia [29]. The difference in prevalence between our study and other previous studies could be partly due to the methodology used, in other previous studies only samples positive based on RBPT were tested with ELISA while our study tested all the samples with iELISA, the sampling frame, this frame was based on the number of livestock seen at time of sampling and may not depict the actual sampling frame of livestock slaughtered at the abattoir and also the study location the abattoir is the largest in the south west Nigeria and collects livestock from various locations across the country. Another important issue is the difference in sensitivity and specificity of serological tests used for screening. This factor contributes to the variations in results among researchers [30]. In small ruminants, seroprevalence of 15.1% and 14.5% was recorded in goat using RBPT and iELISA, respectively, this is quite high compared to previous reports which recorded 2.8% across four States in Nigeria [17]. It is also much higher than prevalence of 2.8% and 1.6% recorded in Somalia and Ethiopia respectively [11]. The likely reasons for the differences could also be due to the fact that the study was conducted in an abattoir which collects livestock from various locations across the country. Seroprevalence of 8.3% and 16.3% was observed among sheep using RBPT and iELISA, respectively. This is higher compared to prevalence of 3.6% [21] and 0.0% [15] reported by these authors. Most of these studies were conducted among herd in the farm, while this study was conducted in an abattoir. The remarkable difference in the prevalence of livestock surveyed is a reflection of the fact that quite a large number of animals in slaughter houses are culled by farmers due to poor performance. The similarity in the rate of infection recorded among the slaughtered livestock shows that trade animal record higher seropositivity compared to those kept in the farms [17].
The explainable reason for higher seroprevalence recorded in the slaughtered livestock could likely be due to the fact that most of the animals slaughtered in the abattoir are gotten from the livestock market and farmers are known to sell animals that are sick or unproductive [31]. More so, there is no prevention and control strategy adopted in Nigeria, contributing to the higher seroprevalence when compared to other studies in other climes where control strategy exists. Higher prevalence of the disease was demonstrated in cattle than in the small ruminants, though the variation is not statistically significant. Similar results were found in Nigeria in which the seroprevalence of the disease was higher in cattle than in goats [15]. The results are also similar to a study carried out in Tanzania which demonstrated higher prevalence in cattle than goats [26]. Although bacteriological analysis was not conducted to determine the strain of Brucella species that was detected in these livestock, the serological test with RBPT was B. abortus and B. melitensis antigen for cattle and small ruminant respectively, B. melitensis is the most pathogenic [32]. However, iELISA test is regarded as the confirmatory serological test as documented by Lopez et al [33]. The seroprevalence recorded in this study suggests that the risk of transmission of brucellosis to abattoir workers is very high. The seroprevalence was more in female cattle than male; however there was no association with Brucella infection. Although most goat sampled were male, the study found high seropositive cases among female goats than male by RBPT; however, there was no association. By iELISA, male goats had higher seroprevalence than the female. In sheep higher prevalence was recorded in female than male by RBPT, but by iELISA, male sheep had higher seroprevalence than female, however this association was not statistically significant. This can be partly explained in terms of sample distribution, most of the sheep sampled were female. Males are kept separately where they are fed well for market value. Their market value is much higher than that of females and they are usually sold when there is a need for cash or are slaughtered during religious ceremonies [34]. Interestingly, it is of importance to note that earlier reports have shown that female animals are more susceptible to Brucella infection and also represents a greater risk of spreading infection [35,36] because they stay longer in the farm and are only culled when they become unproductive. Therefore, having more female animals in the abattoir predisposes the workers to Brucella infection as farmers are known to sell off their female animals which are not doing well, and the most important indicator of not doing well in female animals are reproductive failure and low milk production [37], which are clinical signs of brucellosis in animal.
In this study, the seroprevalence was higher in the adult than the young animals with both serological tests, although this was not statistically significant. This finding is similar to the report of Cadmus et al [38], and Assenga et al [26], but in contrast to Ogugua et al [17] who reported high seroprevalence in young animals. This could be due to increase in concentration of sex hormone which stimulate multiplication of Brucella organism [36]. Again it could also be as a result of the younger animals being resistant to infection, and being able to clear this infection frequently, although re-infection could occur at older age similar to the findings of Ogugua et al [17]. Again, body condition could be a contributing factor to the differences in seroprevalence in livestock [31], on the contrary, this study found no statistical significant association in all the species surveyed. This study also highlights the variations observed in breed specific seroprevalence, in cattle, cross breed recorded the highest prevalence using RBPT, while Adamawa Gudali had the least. With iELISA, the highest breed specific was recorded in Ambala, this was in contrast to the findings of Ogugua et al [38] who reported highest prevalence in Kuri and the reports of Cadmus et al and Junaidu et al [38,39] where the highest prevalence was reported in Bunaji and Sokoto Gudali, respectively. Although one of the factors implicated in conferment of resistance and tolerance is the genetic factor [40]. However, in cattle, the Ambala breed was eight times more likely to be Brucella positive by iELISA compared to the Adamawa Gudali, this shows that breed is associated with brucellosis among cattle in Nigeria [31]. Furthermore, the study found no significant association in both serological tests among goat and sheep breeds. Though, the highest seroprevalence was recorded among Red Sokoto and Uda using RBPT and iELISA, respectively; this is consistent with the reports of Ogugua et al and Junaidu et al [17,41] who reported highest prevalence in Red Sokoto with no significant association between seropositivity and breed of animal, This can equally be explained in terms of sample distribution, most of the goats sampled were Red Sokoto.
Despite the varied seroprevalence of brucellosis in livestock found in the abattoir, these findings confirmed the endemicity of Brucella infection among livestock animals in Nigeria. There is need to establish control strategy for brucellosis among livestock animals by the Federal Ministry of Agriculture. More so, the potential threat the endemicity of brucellosis in animals poses to abattoir workers and other at risk persons calls for the heightened surveillance for brucellosis among febrile patients by the Federal Ministry of Health. A “One Health” collaborative approach in tackling the Brucella problem is hereby recommended.
What is known about this topic
- Brucellosis is endemic in sub-Saharan Africa, with major public health importance;
- Brucellosis is a major neglected zoonosis affecting animals and humans causing abortion and other health conditions;
- Serological methods including RBPT and indirect ELISA techniques have been used successfully in the epidemiological investigations of Brucella infections and brucellosis in animals.
What this study adds
- This study confirmed the presence of Brucella infection in livestock slaughtered in a major abattoir, Lagos State, Nigeria and that seroprevalence varied across species, breed, sex, and age of animals;
- This study showed moderately high seroprevalence of Brucella infections of 17.2%, 15.1% and 8.3% by RBPT, and 15.8%, 14.5% and 23.3% by indirect ELISA among cattle, goats and sheep, respectively slaughtered in a major abattoir, Lagos State, Nigeria;
- The Ambala breed of cattle was found to be more susceptible to Brucella infection being eight times more likely to be Brucella positive compared to the Adamawa Gudali by iELISA.
The authors declare no competing interests.
Ishola Ishola O.O., Ukwueze K.O. and Cadmus S.I.B. made substantial contributions to the design of the study. Ukwueze K.O. designed data collections tools, collected data, conducted the laboratory analyses, analyzed and interpreted the data under the supervision of Ishola, O.O. and Dairo M.D. However, Cadmus S.I.B supervised all aspects of laboratory work and laboratory analyses. Ishola O.O, Awosonya E, Dairo M.D, Cadmus S.I.B revised the manuscript critically for important intellectual content. All the authors have read and approved the final version of the manuscript.
We would specially acknowledge the NFELTP Facilitators, Dr. C.D. Umeokonkwo, Prof. Junaidu Kabir and a host of others for their intellectual contributions to the success of this work.
Table 1: demographic characteristics of livestock slaughtered at Oko-Oba abattoir, Lagos, Nigeria
Table 2: seroprevalence of brucella infection in slaughtered livestock at Oko-Oba abattoir, Lagos, May 2018
Table 3: intrinsic factors associated with seroprevalence of brucella infection using Rose Bengal Plate Test among livestock slaughtered at Oko-Oba abattoir, Lagos
Table 4: intrinsic factors associated with seroprevalence of brucella infection using iELISA among livestock slaughtered at Oko-Oba abattoir, Lagos
- WHO/FAO/OIE. Report of the WHO/FAO/OIE joint consultation on emerging zoonotic diseases. 2004 May; 1-72. Accessed on 14 February 2019.
- Ducrotoy M, Bertu WJ, Matope G, Cadmus SI, Conde-Alvarez R, Gusi AM Welburn S, Ocholi R, Blasco JM, Moriyon I. Brucellosis in Sub-Saharan Africa: Current challenges for management, diagnosis and control. Acta Tropica. 2017; 165. PubMed | Google Scholar
- Sen RB. The state of food and agriculture. Soil Science. 2006; 95(4):284.
- Ducrotoy MJ, Bertu WJ, Ocholi RA, Gusi AM, Bryssinckx W, Welburn S, Moriyón I. Brucellosis as an emerging threat in developing economies: lessons from Nigeria. PLoS Negl Trop Dis. 2014;8(7):e3008. PubMed | Google Scholar
- Muma JB, Godfroid J, Samui KL, Skjerve E. The role of Brucella infection in abortions among traditional cattle reared in proximity to wildlife on the Kafue flats of Zambia. Rev Sci Tech. 2007;26(3):721-730. PubMed | Google Scholar
- Haileselassie M, Kalayou S, Kyule M. Serological survey of bovine brucellosis in barka and arado breeds (Bos indicus) of Western Tigray, Ethiopia. Prev Vet Med. 2010; 94(1-2):28-35. PubMed | Google Scholar
- McDermott JJ, Arimi SM. Brucellosis in sub-Saharan Africa: epidemiology, control and impact. Vet Microbiol. 2002; 90(1-4):111-134. PubMed | Google Scholar
- López GE, Peña S, Escobar GI, Hasan DB, Lucero NE. Serological study of brucellosis in Argentine Creole sheep. Rev Argent Microbiol. 2018; 50(3):285-289. PubMed | Google Scholar
- Pandeya YR, Joshi D, Shah SK. Seroprevalence of brucellosis in different animal species of Kailali district, Nepal. Intern J Infect Dis. 2016;45(1):306. PubMed | Google Scholar
- Ruiz-Villalobos N, Hernandez-Mora G, Bonilla-Montoya R, Romero-Zunlga J, Jimenez-Arias J, Gonzalez-Barrientos R, Barquero-Calvo E, Chacon-Diaz C, Rojas N, Chaves- Olarte E, Guzman-Verri C, Morreno E. Epidemiology of bovine brucellosis in Costa Rica?: Lessons learned from failures in the control of the disease. PLoS One. 2017; 12(8):1-17. PubMed | Google Scholar
- Teshale S, Muhie Y, Dagne A, Kidanemariam A. Seroprevalence of small ruminant brucellosis in selected districts of Afar and Somali pastoral areas of Eastern Ethiopia: The impact of husbandry practice. Rev Med Vet. 2006; 157(11):557-563. PubMed | Google Scholar
- Cadmus SIB, Osikoya IE, Adesokan HK. Brucellosis in trade cattle in Lagos State: An investigation of two abattoirs. Nigerian Vet J. 2009; 29(4):43-46. PubMed | Google Scholar
- Mai HM, Irons PC, Kabir J, Thompson PN. A large seroprevalence surveyof brucellosis in cattle herds under diverse production systems in northern Nigeria. BMC Vet Res. 2012;8:144. PubMed | Google Scholar
- Ayoola MC, Akinseye VO, Cadmus E, Awosanya E, Popoola OA, Akinyemi OO, Perret L, Stack JA, Taylor A, Moriyon I, Cadmus SIB. Prevalence of bovine brucellsis in slaughtered cattle and barriers to better protection of abattoir workers in Ibadan, South-Western Nigeria. Pan Afr Med J. 2017; 8688:1-11. PubMed | Google Scholar
- Stack JA, Cadmus SIB, Ijagbone IF, Oputa, HE, Adesokan HK. Serological survey of brucellosis in livestock animals and workers in Ibadan. Afr J Biomed Res. 2006;9(3). PubMed | Google Scholar
- Kaltungo B, Saidu S, Sackey AKB, Kazeem HM. Sero-evidence of brucellosis in goats in Kaduna North Senatorial District of Kaduna State, Nigeria. Vet Sci. 2013;2013:963673. PubMed | Google Scholar
- Ogugua AJ, Akinseye VO, Ayoola MC, Oyesola OO, Shima FK, Tijani AO, Musa ANA, Adesokan HK, Perret L, Taylor A, Stack JA, Moriyon I, Cadmus SIB. Seroprevalence and risk factors of brucellosis in goats in selected states in Nigeria and the public health implications. Afr J Med Med Sci. 2015;43(1):121-129. PubMed | Google Scholar
- Akinmoladun OI, Adejumo OT. Urban agriculture in metropolitan Lagos: An inventory of potential land and water resources. J. Geog Regional Planning. 2011;4(1):9-19. PubMed | Google Scholar
- Bwala DG, McCrindle C, Fasina FO, Ijagbone I. Abattoir characteristics and seroprevalence of bovine brucellosis in cattle slaughtered at Bodija municipal abattoir, Ibadan, Nigeria. J Vet Med Anim Health. 2015; 7(5):164-168. PubMed | Google Scholar
- Ademola AI. Incidence of fetal wastage in cattle slaughtered at Oko-Oba abattoir and lairage, Agege, Lagos, Nigeria. Medwell Journals. 2010;3(3):54-57. PubMed | Google Scholar
- Boukary AR, Saegerman C, Abatih E, Fretin D, Alambedji Bada R, De Deken R, Adamou Harouna H, Yenikoye A, Thys E. Seroprevalence and potential risk factors for Brucella spp. infection in traditional cattle, sheep and goats reared in urban, periurban and rural areas of Niger. PLoS One. 2013;8(12):e83175. PubMed | Google Scholar
- Kwantes LJ. Ageing of omani small ruminants by permanent incisor growth. Trop Anim Health Prod. 1994 December;26(4):210-212. PubMed | Google Scholar
- Peacock. United States Patent (30) Foreign Application Priority Data. Accessed on 24 April 2019.
- Kashiwazaki Y, Ecewu E, Imaligat JO, Mawejje R, Kirunda M, Kato M, Musoke GM, Ademun RAO. Epidemiology of bovine brucellosis by a combination of Rose Bengal test and indirect ELISA in the five districts of Uganda. Journal Vet Med Sci. 2012;74(11):1417-1422. PubMed | Google Scholar
- OIE. A manual of diagnostic tests and vaccines for terrestrial animals. Office international des epizooties, Paris, France. 2008; 1092-106. Accessed on 21 December 2018.
- Assenga JA, Matemba LE, Muller SK, Malakalinga JJ, Kazwala RR. Epidemiology of Brucella infection in the human, livestock and wildlife interface in the Katavi- Rukwa ecosystem, Tanzania. BMC Vet Res. 2015;11:189. PubMed | Google Scholar
- Ogugua AJ, Akinseye VO, Ayoola MC, Stack J, Cadmus SIB. Risk factors associated with brucellosis among slaughtered cattle: Epidemiological insight from two metropolitan abattoirs in southwestern Nigeria. Asian Pacific Journal of Tropical Disease. 2015;5:9. Google Scholar
- Hesterberga GB, Bagnallb R, Perrettb K, Boschb B, Hornerb R. A serological prevalence survey of Brucella abortus in cattle of rural communities in the province of KwaZulu-Natal, South Africa. J S Afr Vet Assoc. 2008;79(1):15-18. PubMed | Google Scholar
- Jergefa T, Kelay B, Bekana M, Teshale S, Gustafson H, Kindahl H. Epidemiological study of bovine brucellosis in three agro-ecological areas of central Oromiya, Ethiopia. Rev Sci Tech. 2009;28(3):933-943. PubMed | Google Scholar
- Matovic K, Radojicic S. Examination of sensitivity and specificity of some serological tests in diagnostics of bovine brucellosis. Acta Veterinaria. 2008;58(5-6):467-478. PubMed | Google Scholar
- Akinseye VO, Adesokan HK, Ogugua AJ, Adedoyin FJ, Otu PI, Kwaghe AV et al. Sero-epidemiological survey and risk factors associated with bovine brucellosis among slaughtered cattle in Nigeria. Onderstepoort J Vet Res. 2016;83(1)a1002. PubMed | Google Scholar
- United Nations. The state of food and agriculture. 2009. Accessed on 17 December 2018.
- López GE, Pena S, Escobar GI, Hasan DB, Lucero NE. Serological study of brucellosis in Argentine Creole sheep. Rev Argent Microbiology. 2017;30(20):30. PubMed | Google Scholar
- Thys E, Schiere H, Van Huylenbroeck G, Mfoukou-Ntsakala A, Oueadraogo M, Geerts S. Three approaches for the integrated assessment of urban household livestock production systems. Cases from Sub-Saharan Africa. Outlook on Agriculture. 2006;35(1):7-18. PubMed | Google Scholar
- Berhe G, Belihu K, Asfaw Y. Seroepidemiological investigation of bovine brucellosis in the extensive cattle production system of Tigray Region of Ethiopia. Intern J Appl Res Vet Med. 2007;5(2):65-71. PubMed | Google Scholar
- Dinka H, Chala R. Seroprevalence study of bovine brucellosis in pastoral and agro-pastoral areas of East Showa Zone, Oromia Regional State, Ethiopia. Am J Agric Environ Sci. 2009;6(5):508-512. PubMed | Google Scholar
- Ogugua AJ, Akinseye VO, Cadmus EO, Awosanya EA, Alabi PI, Idowu OS, Akinade SA, Dale EI, Perret L, Taylor A, Moriyon I, Cadmus SIB. Prevalence and risk factors associated with bovine brucellosis in herds under extensive production system in southwestern Nigeria. Trop Anim Health Prod. 2018;50(7):1573-1582. PubMed | Google Scholar
- Cadmus SIB, Adesokan HK, Adedokun BO, Stack JA. Seroprevalence of bovine brucellosis in trade cattle slaughtered in Ibadan, Nigeria, from 2004-2006. J S Afr Vet Assoc. 2010;81(1):50-53. PubMed | Google Scholar
- Junaidu AU, Oboegbeluem SI, Salihu MD. Seroprevalence of brucellosis in prison farm in Sokoto, Nigeria. Asian J Epiedmiology. 2008;1(1):24-28. PubMed | Google Scholar
- Barthel R, Feng J, Piedrahita JA, McMurray DN, Templeton JW, Adams LG. Stable transfection of the bovine NRAMP1 gene into murine RAW264.7 cells: Effect on Brucella abortus survival. Infect Immun. 2001;69(5):3110-3119. PubMed | Google Scholar
- Junaidu AU, Daneji AI, Salihu MD, Magaji AA, Tambuwal FM, Abubakar MB, Nawawi H. Seroprevalence of brucellosis in goat in Sokoto, Nigeria. Curr Res J Biol Sci. 2010;2(4):275-277. PubMed | Google Scholar











