Self-reported hypertension as a predictor of chronic health conditions among older adults in Ghana: analysis of the WHO Study on global Ageing and adult health (SAGE) Wave 2
John Tetteh, Kow Entsua-Mensah, Alfred Doku, Sheriff Mohammed, Swithin Mustapha Swaray, Martin Amogre Ayanore, Alfred Edwin Yawson
Corresponding author: Alfred Edwin Yawson, Department of Community Health, University of Ghana Medical School, College of Health Sciences, Korle-Bu, P.O. Box 4236, Accra, Ghana 
Received: 08 Jan 2020 - Accepted: 04 Mar 2020 - Published: 04 May 2020
Domain: Community health,Health Research,Public health
Keywords: Older adults, self-reported hypertension, chronic conditions
©John Tetteh et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: John Tetteh et al. Self-reported hypertension as a predictor of chronic health conditions among older adults in Ghana: analysis of the WHO Study on global Ageing and adult health (SAGE) Wave 2. Pan African Medical Journal. 2020;36:4. [doi: 10.11604/pamj.2020.36.4.21489]
Available online at: https://www.panafrican-med-journal.com//content/article/36/4/full
Research 
Self-reported hypertension as a predictor of chronic health conditions among older adults in Ghana: analysis of the WHO Study on global Ageing and adult health (SAGE) Wave 2
Self-reported hypertension as a predictor of chronic health conditions among older adults in Ghana: analysis of the WHO Study on global Ageing and adult health (SAGE) Wave 2
John Tetteh1,2, Kow Entsua-Mensah2, Alfred Doku2, Sheriff Mohammed3, Swithin Mustapha Swaray2, Martin Amogre Ayanore4, Alfred Edwin Yawson1,&
1Department of Community Health, University of Ghana Medical School, College of Health Sciences, Korle-Bu, P.O. Box 4236, Accra, Ghana, 2National Cardiothoracic Centre, Korle-Bu Teaching Hospital, Accra, Ghana, 3Department of Surgery, University of Ghana Medical School, College of Health Sciences, Korle-Bu, Ghana, 4Department of Health Policy Planning and Management, School of Public Health, University of Health and Allied Sciences, Ho, Ghana
&Corresponding author
Alfred Edwin Yawson, Department of Community Health, University of Ghana Medical School, College of Health Sciences, Korle-Bu, P.O. Box 4236, Accra, Ghana
Introduction: hypertension has been identified as a significant predictor of many chronic health conditions. Body Mass Index (BMI) and Quality of Life (QoL) are key determinants of hypertension especially among elderly populations. In this study, we examined the effect of self-reported hypertension (SRH) on chronic health conditions and quality of life among older adults in Ghana.
Methods: the WHO Study on Global Ageing and Adult Health Wave 2 data for Ghana, collected from 2014 to 2015 was applied in this study. Data for older adults aged 50 years and above were analyzed. Weighted descriptive and inferential analyses were performed using Stata 14. We predicted any potential associations between SRH and chronic health conditions using a corrected chi-square and Coarsened Exact Matching with adjusted odds ratios.
Results: the prevalence of SRH among older adults in Ghana was 15.8%. This was significantly associated with sex, marital status, religion, place of residence, working status, location/region, health status BMI, and quality of life (QoL). In all, older adults with poor health status, obese state and high QoL had 3.15, 2.17 and 2.76 odds of SRH respectively [AOR(95%CI)p-value=3.15(1.65-6.02)0.001, 2.17(1.31-3.59)0.003 and 2.76(1.04-7.31)0.041)]. In addition, older adults with SRH were at increased risk of reporting chronic conditions such as stroke, angina, diabetes and cataract.
Conclusion: overall, a key observation from this analysis is that SRH (and not only clinically diagnosed hypertension) is significantly associated with co-morbidities. In Ghana, older adults with SRH have increased risk of co-morbidities including diabetes, stroke, angina, and cataract. Interventions to improve the awareness and early detection of hypertension at the population level is key. Controlling hypertension at the population level will reduce prevalence of chronic conditions and increased protection.
Hypertension is a major risk factor for cardiovascular diseases worldwide [1]. The 2017 Global Burden of Disease (GBD) indicated that hypertension was an important public-health challenge worldwide and was estimated to affect 1.56 billion individuals by 2025 with an increased global prevalence of 60% [1, 2]. Hypertension is a chronic multifactorial disease whose detection is often delayed due to its slow and silent progression [2]. However, this asymptomatic chronic disease in most people requires optimal control usually through disciplined adherence to prescribed medications. Without optimal control, there is increased risk of developing cardiovascular, cerebrovascular and renal complications [3].
In sub-Saharan Africa (SSA), the prevalence of hypertension and pre-hypertension is high, with an estimated prevalence rate of 25.9%. This, however, differs by population group [4]. The 2014 Ghana Demographic Health Survey (GDHS) reported a hypertension prevalence rate of 13% among adults [5] in the reproductive age, while the WHO Study on Global AGEing and Adult Health (SAGE), Wave 1 in Ghana reported SRH prevalence rate of 14.2% among older adults 50 years and above [6]. Age is universally associated with hypertension, and in Africa, the greatest burden of hypertension is borne by persons 50 years and above [7]. Increasing age, is a major cause of reduction in the quality of life (QoL) among older adults. [8]. The QoL which is an individual´s perception of happiness and satisfaction with life as well as position in life and value systems in relation to expectations, values and concerns with physical health may be severely compromised [9]. Low QoL affects the psychological well being and socio-demographic functions [10]. Invariably, QoL is an important predictor of treatment outcomes among older adults with hypertension and QoL among older adults with hypertension has been shown to be worse than those without hypertension [10, 11].
However, hypertension among older adults may not be detected early or may not be adequately managed [12]. The consequences of this on the health and social well being of these older persons may be dire. Imperatively, measures needed to be taken for early diagnosis of hypertension and to prevent its complications is essential at the population level, considering that the prevalence of hypertension is estimated to increase [2]. Globally, there is substantial connection between hypertension and other chronic health condition which reflect in their etiology and disease mechanisms. Hypertension is common among patients with diabetes [13] and stroke [14] . It has been observed in previous studies that 51% of stroke deaths are attributable to hypertension [15]. Hypertension is associated with other chronic conditions that affect the QoL of older persons including eye conditions such as cataract. This impairs the daily functioning of the older adults and affects their quality of life [16]. This analysis determined the prevalence of SRH, predictors of hypertension and quantified the effect of SRH on stroke, angina, diabetes and cataract among older adults in Ghana.
Study setting: the study was conducted in Ghana with an estimated population of 29,694,793 as at the year 2018 [17, 18] and 5,865 established health facilities across the country [19].
Data and study participants: WHO Study on Global AGEing and Adult Health (SAGE) Wave 2 for Ghana dataset was used in this study. SAGE is a longitudinal data on the health and well-being of adult populations as well as the ageing process conducted by WHO. Between 2014 and 2015, SAGE Wave 2 country studies were conducted in six countries; China, Ghana, India, Mexico, Russian Federation and South Africa [20]. Two target populations were measured in SAGE Wave 2. A large sample of persons aged 50 years and older (focus group for SAGE) and a smaller comparative sample of persons in reproductive age group (aged 18-49 years). Households were classified into mutually exclusive categories where one or more persons aged 50 years and older were selected from these households and classified as 50+ household. The presence of one person aged 18-49 years in a household classified as an 18-49 household. In the older households, all persons aged 50 years and older were invited to participate whist proxy respondents were identified for respondents who were unable to respond for themselves. Multistage cluster sampling was used for Ghana Wave 2 with 250 Primary Sample Units and 20 strata [6, 20]. Detailed study design and procedure for data collection adopted in SAGE wave 2 is well described by Kowal et al. (2012) [21]. A total of 4,735 persons, comprising older adults (50+ years) and persons in their reproductive age (18-49 years) were interviewed. In line with the aim of the study, data for persons below 50 years and missing responses for SRH were excluded. A final sample of 2,335 adults aged 50 years and above were included in this study.
Dependent variables: self-reported hypertension (SRH) was considered the primary outcome variable.. Co-morbidities including stroke, angina, diabetes and cataract were also considered as outcome variables. The SRH was defined ‘as ever been told by a doctor or health care professional that you have high blood pressure (hypertension)´.
Independent variable: from the original dataset, sex, marital status, religion, educational attainment, residence, region of residence and employment status were extracted for the sociodemographic variables. BMI was estimated from a measured weight (kg) and height (metres) and categorized into <18.5, 18.5-24.9, 25-29.9 and 30 and above as underweight, normal, overweight and obesity respectively. Perceptions on QoL and well-being using the 8-item WHO Quality of Life measure (WHOQoL) from standard questions were used to grade level of life satisfaction. The 8-standard questions were recoded as satisfied=1 and not satisfied=0. An index variable was created for the recoded 8-standard questions with scores ranging from 0-8. These scores (continuous variables) were further reclassified into 0, 1-5, 6-7 and 8 as poor satisfaction, low satisfaction, moderate satisfaction and very satisfied respectively.
Data analysis: descriptive statistics involved a weighted two-way observational row percentage table involving independent variables associated with SRH. This was done with a corrected chi-square value taking into consideration the design nature of SAGE Wave 2. The second step of analysis involved inferential statistics involving logistics regression to assess risk factors that are associated with SRH. Coarsened Exact Matching (CEM) method of analysis was carried to improve and reduce imbalances in estimating causal effects. This was conducted to control for some or all of the potentially confounding influence of pretreatment control variables by reducing imbalances between the treated and control groups. This Monotonic Imbalance Bounding (MIB) matching method which balances between the treated (SRH) and control groups (non-SRH), was employed to adjustthe influence of the imbalance of one variable on the maximum imbalance of any other [22]. In this analysis, SRH predictors identified included; Age, Place of residence, Region, Health status, BMI and QoL. These were modeled to reduce the imbalances between them (Table 1).
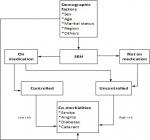
Overall 76% of imbalance existed among the independent variables before matching. However, after CEM matching, imbalances reduced to almost 0% (5.63E-16) (Table 1). After preprocessing the data with CEM, sensitivity analysis was applied involving Logistics regression, weighted with CEM estimations to estimate the risk of SRH on stroke, diabetes, angina and cataract adjusting for non-predictors of SRH which includes; sex, marital status, religion and working status. The concept and process involved in conducting this analysis is demonstrated in Figure 1; that older adults with SRH can either be controlled with medication or uncontrolled without medication. The risk of stroke, angina, diabetes and cataract can be either low or high among older adults with SRH in relation to management of the condition, whilst the demographic predictors can also directly affect the health condition.
Ethical requirements: the SAGE study was approved by the World Health Organization's Ethical Review Board (reference number RPC149) and the Ethical and Protocol Review Committee, College of Health Sciences, University of Ghana, Accra, Ghana. Written informed consent was obtained from all study respondents.
Data on a total of 2,335 older adults 50 years and above were analyzed (Table 1). The study found a prevalence of 15.8% for SRH among the older adults in Ghana. Sex differential in SRH existed and was relatively higher among females (20.3% vs. 11.6%). Those in the age group 60-69 years had the highest prevalence of SRH (18.5%). Older adults who reported marital status as separated, had the highest prevalence of SRH (22.5%). In addition, rural-urban disparities existed in the prevalence of SRH and was relatively higher among urban residents (23.1% vs. 8.1%) , while unemployed older adults had relatively higher SRH , than those who were working (19.8% vs. 13.2%) In terms of geographical location, older adults living in the Volta region had the highest risk of SRH (51%), compared with those living in the other nine administrative regions in Ghana. Unsurprisingly, poor perception of health status was associated with a higher SRH among the older adults (28.7%) and SRH was highest among obese individuals (34.2%) as compared with other BMI categories (Table 2). Older adults belonging to no identifiable religion had the highest prevalence of SRH (18.3%)
In all, differences in SRH among older adults in Ghana were significantly associated with sex, marital status, religion, place of residence, employment status, geographical location/region of residence, health status and BMI (Table 2). Interestingly, among the 329 older adults who self-reported hypertension, only 94 (27.4%) were on antihypertensive medications. Older people residing in rural areas had a 43% chance of developing hypertension compared with those who lived in urban areas [aOR(95%CI)=0.43(0.29-0.62)0.000]. Again, older adults aged 70-79 were 88% more likely to have compared with the relatively younger individuals [aOR(95%CI)=1.88(1.07-3.21)0.027]. The other nine administrative regions had a lesser risk of SRH compared with Greater Accra region (as in Table 3, Table 3 (suite)). Older adults who rated their health status as bad were 3.2 times more likely to have SRH [aOR(95%CI)p-value=3.15(1.65-6.02)0.000] whilst obese older adults were 2.3 times more likely to self-report hypertension [aOR(95%CI)p-value=2.25(1.32-3.85)0.003]. Older adults who were highly satisfied with their life were 3.0 times more likely to self-report hypertension [aOR(95%CI)p-value=3.01(1.09-8.32)0.034] as compared with older adults with poor life satisfaction i.e. the higher the QoL, the higher the risk of SRH (Table 3, Table 3 (suite)).
In our analysis the prevalence of the selected chronic conditions among older adults with SRH were stroke (2.0%), angina (2.6%), diabetes (3.8%) and cataract (10.0%). Controlling for significant factors associated with SRH and adjusting for; sex, marital status, religion and working status, self-reported hypertension was a significant predictor of stroke, angina, diabetes and cataract among older adults in Ghana (p-value<0.05) as demonstrated in in Table 4. Older adults with SRH had an increased odds for stroke, angina, diabetes and cataract, with significant adjusted odds of 30.9, 4.6, 3.6 and 2.0 respectively compared with those without SRH [aOR(95%CI)=30.85(9.34-102.1), 4.56(1.53-13.55), 3.59(1.46-8.84) and 2.03(1.19-3.46)] (Table 4).
Prevalence and risk factors associated with self-reported hypertension among older adults: this analysis found the prevalence of self-reported hypertension (i.e. hypertensives who are aware of their condition) to be 15.8% among older adults in Ghana. This is relatively high as compared with what was reported in WHO SAGE Wave 1 (2008-2009), which reported a prevalence rate of 14.0% [6]. Hypertension is of major public health concern [23-26] and a major risk factor for cardiovascular diseases among adults [23]. A study conducted in Ghana among adults aged 25 years and above identified a 28.3% prevalence rate for hypertension [26] whereas a systematic review identified a range of 19% to 48% prevalence rate of hypertension across all age groups in Ghana [23]. The Ghana Demographic and Health (GDHS) survey of 2014 (coterminous with data collection for WHO SAGE Wave 2) reported a prevalence rate of 13% among men and women in the reproductive age bracket of 15-49 years [5]. This is slightly less than what SAGE wave 2 found, probably due to the relatively younger population used by the GDHS. The identified prevalence of 15.8% is well within that identified in the United States of 13%-19% but lesser than the 24.1% reported by Malta et al. among the older adults population of Brazil [2, 27].
Sex differences were identified to have a statistically significant association with SRH, with a female preponderance (20.3% vs. 11.6%). . . Sex differences in the reported rates of hypertension are well documented, males have a higher prevalence for hypertension compared with females of the same age until the 6th decade when females begin to report more [28-30]. Sex differences in hypertension is postulated to be a result of the role of the immune system in mediating sex differences in hypertension where kidneys, the renin-angiotensin system, and developmental programming known to play a role [30,31]. Akin to findings from previous studies, this analysis demonstrated increasing prevalence of SRH with increasing age; adults aged 60 years and above experienced a higher rate SRH (18.5) [24, 26, 28, 32].
In addition, marital status is associated with higher SRH, older adult Ghanaians who were separated from their spouses experienced a higher prevalence rate for hypertension, compared with married older adults. Marriage may afford some protective effect on moderating lifestyle habits such as alcohol intake and poor dietary habits. However, this finding contradicts an observation that single individuals are better off in blood pressure profile than unhappy married individuals [33]. Intuitively, emotions and stressors in such a union may increase the risk of hypertension. It has been established that religion improves QoL and lowers risk for developing hypertension [34]. Aligned with this, religion was found to be significantly associated with SRH and that older adults without any form of religious affiliation reported a higher prevalence of SRH (18.3%). Religion, belief systems and health have been demonstrated to be related in all population groups and is statistically correlated to the prevalence of hypertension [35, 36].
Generally, there are inconsistencies in rural-urban disparities in the prevalence of SRH. In our analysis, urban dwellers had relatively higher SRH while rural-dwelling adults were at lower risk and had a decreased predictive probability of SRH. This finding is in consistent with the findings of several publications [37-40]. At the same time, it contradicts the findings of other published work where the burden of hypertension among the older adults is significantly higher in rural areas [12, 41-44]. In Ghana, the relative higher prevalence of SRH in urban areas is not surprising. Rural dwellers are more active, are engaged in more physically tasking occupations and have lower prevalence of obesity and inequities in access to health providers [19]. Conversely, urban dwellers may have increased access and exposure to information (traditional and social media) and better awareness of hypertension in the urban areas. This is buttressed by the finding that the most urbanized region, Greater Accra, had the highest prevalence of SRH in Ghana.
In this analysis, adults who were not working reported a greater prevalence of SRH which was similar to a study conducted in Japan where job strain was significantly related to hypertension [45]. This, however, contradicts a retrospective cohort study conducted among 13 European countries where no association was found between working status and hypertension among adults, [46]. Unsurprisingly adults who were within the fifth wealth quintile (richest) had the highest SRH, more likely to be due to increased access, more exposure to information and healthcare. This finding is in keeping with findings by other authors who found that adults from the highest wealth quintile were significantly more likely to have hypertension [47, 48]. Individual’s health status was observed to have a significant association with SRH, such that adults with self-rated bad health conditions were observed to experience a higher SRH and were more likely to be hypertensive. Similarly obese older adults were observed to report high prevalence for hypertension, in keeping with findings from other studies [49-51].
Effect of Self-reported hypertension on stroke, angina, diabetes and cataract among older adults in Ghana: this analysis demonstrated clearly that, self-reported hypertension significantly predicts the presence of chronic conditions such as stroke, angina, diabetes and cataract among older adults (in Ghana). We found specifically that self-reported hypertension in older adults increased the risk of stroke, angina, diabetes and cataract compared with older persons without SRH. Other studies [13, 15, 16, 52-57] corroborate this observation. The relationship between hypertension and other chronic condition is well known globally; many people with diabetes have hypertension [13, 52, 53] which increases the risk of stroke [15] and as well as increased risk of cataract [58]. In addition, angina can also be related to hypertension due to the pressure at which the heart has to exert to pump blood [59]. Overall, a key observation from this analysis is that SRH (and not only clinically diagnosed hypertension) is significantly associated with hypertension.
Limitations: this analysis used only self-reporting hypertension by older adults. A combination of other methods like consecutive measurement to confirm hypertension could probably have yielded additional information.
Overall, a key observation from this analysis is that SRH (and not only clinically diagnosed hypertension) is significantly associated with co-morbidities. In Ghana, older adults with SRH have increased risk of co-morbidities including diabetes, stroke, angina, and cataract. Interventions to improve the awareness and early detection of hypertension at the population level is key. Controlling hypertension at the population level will garner reduction in prevalence of chronic conditions and increased protection.
What is known about this topic
- 14.2% self-reported hypertension exist among older adults 50 years and above;
- The 2014 Ghana Demographic Health Survey reported a hypertension prevalence rate of 13% among adults;
- There is substantial connection between hypertension and other chronic health condition.
What this study adds
- The prevalence of self-reported hypertension among older adults in Ghana was 15.8%;
- Self-reported hypertension is associated with sex, marital status, religion, place of residence, working status, location/region, health status, BMI, and quality of life;
- Older adults with self-reported hypertension were at increased risk of reporting chronic conditions such as stroke, angina, diabetes and cataract.
The authors declare no competing interests.
JT conceptualized the study and sought approval for access to the SAGE wave 2 data. AEY is member of the WHO SAGE Wave 2 Team in Ghana. JT, KEM and AEY undertook the statistical analysis. JT, SMS and SM drafted the initial manuscript. KEM, AD, AEY and MAA read and provided intellectual content revisions and suggestions for clarity and precision on subject matter. All authors read and approved the final review manuscript.
Authors are grateful to WHO SAGE wave 2 Ghana Team for providing the data for this research work. Special acknowledgement also goes to Miss Agustina Dede Tetteh for her inspirations and support during the research work.
Table 1: CEM weighted balance report before and after matching
Table 2: demographic characteristics of older adults with self-reported hypertension in Ghana n (%) Weighted %
Table 3: demographic risk factors associated with self-reported hypertension in the sample population
Table 3 (suite): demographic risk factors associated with self-reported hypertension in the sample population
Table 4: effect of self-reported hypertension on selected chronic conditions (stroke, angina, diabetes and cataract) in older adults in Ghana
Figure 1: framework defining the impact of self-reported hypertension on chronic conditions among older adults
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005 Jan 15-21;365(9455):217-23. PubMed | Google Scholar
- Dorans KS, Mills KT, Liu Y, He J. Trends in Prevalence and Control of Hypertension According to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) Guideline. J Am Heart Assoc. 2018 Jun 1;7(11). pii: e008888. PubMed | Google Scholar
- Robles NR, Macias JF. Hypertension in the elderly. Cardiovasc Hematol Agents Med Chem. 2015;12(3):136-45. PubMed | Google Scholar
- Guwatudde D, Nankya-Mutyoba J, Kalyesubula R, Laurence C, Adebamowo C, Ajayi I et al. The burden of hypertension in sub-Saharan Africa: a four-country cross sectional study. BMC Public Health. 2015 Dec 5;15:1211. PubMed | Google Scholar
- GSS, GHS, ICF. Ghana Demographic and Health Survey 2014. Rockville, Maryland, USA: GSS, GHS, and ICF International. 201.
- Biritwum RB, Yawson AE, Mensah G. Ghana: Study on global AGEing and adult health (SAGE) Wave 1 National Report. ResearchGate. Epub ahead of print. May 2015.
- Pinto E. Blood pressure and ageing. Postgrad Med J. 2007 Feb;83(976):109-14. PubMed | Google Scholar
- WHO. Proposed working definition of an older person in Africa for the MDS Project. Accessed 21st November 2018.
- Mazaheri M. The QoL-DASS Model to Estimate Overall Quality of Life and General Subjective Health. Iran J Psychiatry. 2011 Winter;6(1):43-6. PubMed | Google Scholar
- Ha NT, Duy HT, Le NH, Khanal V, Moorin R. Quality of life among people living with hypertension in a rural Vietnam community. BMC Public Health. 2014 Aug 11;14:833. PubMed | Google Scholar
- Trevisol DJ, Moreira LB, Kerkhoff A, Fuchs SC, Fuchs FD. Health-related quality of life and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2011 Feb;29(2):179-88. PubMed | Google Scholar
- Chinnakali P, Mohan B, Upadhyay RP, Singh AK, Srivastava R, Yadav K. Hypertension in the Elderly: Prevalence and Health Seeking Behavior. N Am J Med Sci. 2012 Nov;4(11):558-62. PubMed | Google Scholar
- de Boer IH, Bangalore S, Benetos A, Davis AM, Michos ED, Muntner P et al. Diabetes and Hypertension: A Position Statement by the American Diabetes Association. Diabetes Care. 2017; 40: 1273-1284. PubMed | Google Scholar
- O´Rourke MF. Frederick Akbar Mahomed. Hypertension. 1992 Feb;19(2):212-7. PubMed | Google Scholar
- Gaciong Z, Siński M, Lewandowski J. Blood Pressure Control and Primary Prevention of Stroke: Summary of the Recent Clinical Trial Data and Meta-Analyses. Curr Hypertens Rep. 2013 Dec;15(6):559-74. PubMed | Google Scholar
- Yawson AE, Ackuaku-Dogbe EM, Seneadza NA, Mensah G, Minicuci N, Naidoo N et al. Self-reported cataracts in older adults in Ghana: sociodemographic and health related factors. BMC Public Health. 2014 Sep 12;14:949. PubMed | Google Scholar
- GhanaWeb. Background Information about the Country Ghana. Accessed 12 November 2018.
- World population review. Ghana Population 2020 (Live) (Demographics, Maps, Graphs). Accessed 12 November 2018.
- Ministry of Health. THE Health Sector in Ghana facts and figures. Accessed 12 November 2018.
- WHO. Health statistics and information systems: SAGE wave 0, 1, 2 & 3. Accessed 12 November 2018.
- Kowal P, Chatterji S, Naidoo N, Biritwum R, Fan W, Lopez Ridaura R et al. Data Resource Profile: The World Health Organization Study on global AGEing and adult health (SAGE). Int J Epidemiol. 2012 Dec;41(6):1639-49. PubMed | Google Scholar
- Satefano MI, Gary K, Giuseppe P. CEM: Coarsened Exact Matching Software. Accessed 12 November 2018.
- Bosu WK. Epidemic of hypertension in Ghana: a systematic review. BMC Public Health. 2010 Jul 14;10:418. PubMed | Google Scholar
- Khajedaluee M, Hassannia T, Rezaee A, Ziadi M, Dadgarmoghaddam M. The prevalence of hypertension and its relationship with demographic factors, biochemical, and anthropometric indicators: A population-based study. ARYA Atheroscler. 2016 Nov; 12(6): 259–265. PubMed | Google Scholar
- Addo J, Smeeth L, Leon DA. Hypertensive Target Organ Damage in Ghanaian Civil Servants with Hypertension. PLoS One. 2009 Aug 18;4(8):e6672. PubMed | Google Scholar
- Amoah AG. Hypertension in Ghana: a cross-sectional community prevalence study in greater Accra. Accessed 14 November.
- Malta DC, Bernal RTI, Andrade SSCA, Silva MMAD, Velasquez-Melendez G. Prevalence of and factors associated with self-reported high blood pressure in Brazilian adults. Rev Saude Publica. 2017 Jun 1;51(suppl 1):11s. PubMed | Google Scholar
- Yoon SS, Gu Q, Nwankwo T, Wright JD, Hong Y, Burt V. Trends in blood pressure among adults with hypertension: United States, 2003 to 2012. Hypertension. 2015 Jan;65(1):54-61. PubMed | Google Scholar
- James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J et al. 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults: Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014 Feb 5;311(5):507-20. PubMed | Google Scholar
- Gillis EE, Sullivan JC. Sex Differences in Hypertension: Recent Advances. Hypertension. 2016 Dec;68(6):1322-1327. PubMed | Google Scholar
- Maranon R, Reckelhoff JF. Sex and Gender Differences in Control of Blood Pressure. Clin Sci (Lond). 2013 Oct;125(7):311-8. PubMed | Google Scholar
- Lionakis N, Mendrinos D, Sanidas E, Favatas G, Georgopoulou M. Hypertension in the elderly. World J Cardiol. 2012 May 26;4(5):135-47. PubMed | Google Scholar
- Miranda H. Happy Marriage, Better Blood Pressure (WebMD). Accessed 25th January 2019.
- Idler EL, McLaughlin J, Kasl S. Religion and the Quality of Life in the Last Year of Life. J Gerontol B Psychol Sci Soc Sci. 2009 Jun;64(4):528-37. PubMed | Google Scholar
- Meng Q, Xu Y, Shi R, Zhang X, Wang S, Liu K et al. Effect of religion on hypertension in adult Buddhists and residents in China: A cross-sectional study. Sci Rep. 2018 May 29;8(1):8203. PubMed | Google Scholar
- Koenig HG. Religion, Spirituality, and Health: The Research and Clinical Implications. ISRN Psychiatry. 2012 Dec 16;2012:278730. PubMed | Google Scholar
- Li G, Hu H, Dong Z, Xie J, Zhou Y. Urban and Suburban Differences in Hypertension Trends and Self-Care: Three Population-Based Cross-Sectional Studies from 2005-2011. PLoS One. 2015 Feb 9;10(2):e0117999. PubMed | Google Scholar
- Li J, Shi L, Li S, Xu L, Qin W, Wang H. Urban-rural disparities in hypertension prevalence, detection, and medication use among Chinese Adults from 1993 to 2011. Int J Equity Health. 2017 Mar 14;16(1):50. PubMed | Google Scholar
- Adediran OS, Okpara IC, Adeniyi OS. Hypertension prevalence in an urban and rural area of Nigeria. J Med Med Sci. 2013; 4: 149-154. Google Scholar
- Adebayo RA, Balogun MO, Adedoyin RA, Obashoro-John OA, Bisiriyu LA, Abiodun OO. Prevalence of hypertension in three rural communities of Ife North Local Government Area of Osun State, South West Nigeria. Int J Gen Med. 2013 Dec 3;6:863-8. PubMed | Google Scholar
- Roy A, Praveen PA, Amarchand R, Ramakrishnan L, Gupta R, Kondal D et al. Changes in hypertension prevalence, awareness, treatment and control rates over 20 years in National Capital Region of India: results from a repeat cross-sectional study. BMJ Open. 2017 Jul 12;7(7):e015639. PubMed | Google Scholar
- Akpan EE, Ekrikpo UE, Udo AI, Bassey BE. Prevalence of Hypertension in Akwa Ibom State, South-South Nigeria: Rural versus Urban Communities Study. Int J Hypertens. 2015;2015:975819. PubMed | Google Scholar
- Daştan İ, Erem A, Çetinkaya V. Urban and rural differences in hypertension risk factors in Turkey. Anatol J Cardiol. 2017 Jul; 18(1): 39–47. PubMed | Google Scholar
- Wang J, Sun W, Wells GA, Li Z, Li T, Wu J et al. Differences in prevalence of hypertension and associated risk factors in urban and rural residents of the northeastern region of the People´s Republic of China: A cross-sectional study. PLoS One. 2018 Apr 5;13(4):e0195340. PubMed | Google Scholar
- Tsutsumi A, Kayaba K, Tsutsumi K, Igarashi M; Jichi Medical School Cohort Study Group. Association between job strain and prevalence of hypertension: a cross sectional analysis in a Japanese working population with a wide range of occupations: the Jichi Medical School cohort study. Occup Environ Med. 2001 Jun;58(6):367-73. PubMed | Google Scholar
- Rumball-Smith J, Nandi A, Kaufman JS. Working and hypertension: gaps in employment not associated with increased risk in 13 European countries, a retrospective cohort study. BMC Public Health. 2014 May 30;14:536. PubMed | Google Scholar
- Tareque MI, Koshio A, Tiedt AD, Hasegawa T. Are the Rates of Hypertension and Diabetes Higher in People from Lower Socioeconomic Status in Bangladesh? Results from a Nationally Representative Survey. PLoS One. 2015 May 27;10(5):e0127954. PubMed | Google Scholar
- Harshfield E, Chowdhury R, Harhay MN, Bergquist H, Harhay MO. Association of hypertension and hyperglycaemia with socioeconomic contexts in resource-poor settings: the Bangladesh Demographic and Health Survey. Int J Epidemiol. 2015 Oct;44(5):1625-36. PubMed | Google Scholar
- Jiang S-Z, Lu W, Zong X-F, et al. Obesity and hypertension. Exp Ther Med. 2016; 12: 2395-2399. PubMed | Google Scholar
- Re RN. Obesity-Related Hypertension. Ochsner J. 2009 Fall;9(3):133-6. PubMed | Google Scholar
- Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015 Mar 13;116(6):991-1006. PubMed | Google Scholar
- Cheung BMY, Li C. Diabetes and Hypertension: Is There a Common Metabolic Pathway? Curr Atheroscler Rep. 2012 Apr;14(2):160-6. PubMed | Google Scholar
- Medical News Today. The link between diabetes and hypertension. Accessed 8th May 2019.
- WHO. Ghana country assessment report on ageing and health. Accessed 8th May 2019.
- Tai TY, Chuang LM, Chen CJ, Lin BJ. Link Between Hypertension and Diabetes Mellitus Epidemiological Study of Chinese Adults in Taiwan. Diabetes Care. 1991 Nov;14(11):1013-20. PubMed | Google Scholar
- Vitale C, Kaski JC. Microvascular angina and systemic hypertension. E-J Cardiol Pract; 14. Accessed 8th May 2019.
- Phaswana-Mafuya N, Peltzer K, Crampin A, Ahame E, Sokhela Z. Prevalence of Self-Reported Diagnosed Cataract and Associated Risk Factors among Elderly South Africans. Int J Environ Res Public Health. 2017 Dec 6;14(12). pii: E1523. PubMed | Google Scholar
- Yu X, Lyu D, Dong X, He J, Yao K. Hypertension and Risk of Cataract: A Meta-Analysis. PLoS One. 2014 Dec 4;9(12):e114012. PubMed | Google Scholar
- Sayeed SA. Is angina related to high blood pressure? (Sharecare). Accessed 8th May 2019.