Clinicopathological associations and prognostic values of IDH1 gene mutation, MGMT gene promoter methylation, and PD-L1 expressions in high-grade glioma treated with standard treatment
Julius July, Diana Patricia, Pricilla Yani Gunawan, Handrianto Setiajaya, Teridah Ernala Ginting, Teguh Pribadi Putra, Zerlina Wuisan, Dini Budhiarko, Najmiatul Masykura, Gintang Prayogi, Ahmad Rusdan Utomo, Steven Tandean, Michael Lumintang Loe
Corresponding author: Julius July, Department of Neurosurgery, Medical Faculty of Universitas Pelita Harapan, Neuroscience Centre of Siloam Hospital Lippo Village, Tangerang, Indonesia 
Received: 06 Jul 2020 - Accepted: 29 Jul 2020 - Published: 20 Aug 2020
Domain: Molecular Biology,Neuro-oncology,Neurosurgery
Keywords: IDH1 mutation, MGMT methylation, PD-L1, anaplastic astrocytoma, glioblastoma multiforme
©Julius July et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Julius July et al. Clinicopathological associations and prognostic values of IDH1 gene mutation, MGMT gene promoter methylation, and PD-L1 expressions in high-grade glioma treated with standard treatment. Pan African Medical Journal. 2020;36:309. [doi: 10.11604/pamj.2020.36.309.24831]
Available online at: https://www.panafrican-med-journal.com//content/article/36/309/full
Research 
Clinicopathological associations and prognostic values of IDH1 gene mutation, MGMT gene promoter methylation, and PD-L1 expressions in high-grade glioma treated with standard treatment
Clinicopathological associations and prognostic values of IDH1 gene mutation, MGMT gene promoter methylation, and PD-L1 expressions in high-grade glioma treated with standard treatment
Julius July1,2,&, Diana Patricia3, Pricilla Yani Gunawan4, Handrianto Setiajaya5, Teridah Ernala Ginting2, Teguh Pribadi Putra6, Zerlina Wuisan6, Dini Budhiarko6, Najmiatul Masykura6, Gintang Prayogi6, Ahmad Rusdan Utomo6, Steven Tandean7, Michael Lumintang Loe7
&Corresponding author
Introduction: the objective was to evaluate the impact of IDH1 R132H mutation, MGMT methylation and PD-L1 expression in high grade glioma that received standard therapy (surgery, radiation and chemotherapy) to overall survival (OS).
Methods: this is a retrospective study of 35 high grade glioma cases. Genotyping of IDH1 gene alteration on the mutation hotspot R132 (Sanger sequencing method with Applied Biosystems 3500 Genetic Analyzer), EZ DNA Methylation-Gold kit (Zymo Research) is used to study the methylation, Cell line BT549 (ATCC HTB-122) and HCT-116 (ATCC CCL-247) were used as unmethylated control and partially methylated control respectively. Anti-human PD-L1 antibody clone E1L3N® from Cell Signalling Technology (USA) and Rabbit XP® were used to see PDL-1 expression.
Results: anaplastic astrocytoma cases had more MGMT promoter methylation (50%) than glioblastoma multiforme (GBM) (20%), more IDH1 R132H mutation (42%) than GBM (4.3%). Immunohistochemistry tumor proportion score method (TPS) identified 17% and 8.7% were PD-L1 positive in AA and GBM groups, respectively. Cases with IDH1 R132H mutation and MGMT methylation still showed better OS although with high PD-L1 expression.
Conclusion: IDH1 R132H mutation and MGMT methylation were good prognostic markers. High expression of PD-L1 apparently might not indicate poor overall survival in the presence of IDH1 R132 mutation and MGMT methylation.
Gliomas are the most common central nervous system (CNS) tumors, which are classified into groups including astrocytic, oligodendroglial, oligoastrocytic and other tumors [1, 2]. Prior to 2016, WHO defined grades of malignancy based on tumors´ histology. Grade I tumors are usually well-circumscribed with a relatively good prognosis and prolonged survival [3]. Diffuse infiltrative astrocytoma is defined as grade 2, and those which show anaplasia and mitotic activity is defined as grade III (anaplastic astrocytoma). In addition, tumors that show microvascular proliferation and/or necrosis such as glioblastoma multiforme (GBM) are classified as grade IV. Since 2016, WHO has included both histological and molecular criteria for CNS tumors in order to achieve accurate diagnoses [1, 4, 5]. Glioblastoma multiforme (GBM) constitutes a lethal diagnosis, with a median survival time of 14-16 months, while 36 months is considered as long-term survival. A 26%-33% cases with two-year survival rate was achieved under optimized standard therapy for GBM includes surgical resection, followed by radiation and co-administration of temozolomide (TMZ), an oral alkylating agent [6, 7]. Surgical resection is the most effective way to increase survival of GBM patients. However, it is difficult to do it because these tumors are frequently invasive to highly specialized brain areas, such as those involved in the control of speech, motor function and senses [8, 9]. Beside WHO grading system [4], clinical finding, neurologic performance, tumour location [10] and molecular alterations [11], are used to predict the prognosis of patients.
Malignant tumor progression is accompanied by an altered molecular phenotype driven by gene mutation, DNA promoter methylation and chromosome codeletion. Novel biomarkers such as isocitrate dehydrogenase 1 gene (IDH1) and O6-methylguanine-DNA-methyltransferase (MGMT) gene methylation status have been proposed as useful tool to predict prognosis and survival of glioma patients [11]. IDH1 gene is an enzyme regulating cellular metabolism, epigenetic regulation, redox states, and DNA repair. Somatic mutation at codon 132 of IDH1 gene, is prevalent in WHO grade II or III gliomas and in the secondary glioblastomas, and it is associated with better outcome than those patients with IDH1 wild-type [12]. Soon after the discovery, the IDH1/2 mutations are associated with a relatively prolonged patient survival for glioma and glioblastoma in clinics, and has been a rationale for drug target. In addition, primary and secondary glioblastomas have been classified based on these IDH mutation entities [2, 4]. MGMT is a DNA repair protein, found in human and many prokaryotic organisms. The epigenetic silencing of the MGMT gene by promoter hypermethylation leads to loss of MGMT protein expression thus limiting the activity of glioma repair function. Active MGMT protein removes the alkyl adducts, preventing the crosslink formation, thereby causing the resistance to alkylating drugs [13, 14]. The methylation status of MGMT, rather than the protein expression itself, has been one predictor of alkylating drugs susceptibility and long-term survival in glioma patients [15-17].
One of the mechanisms for immune system against tumor is the generation of cytotoxic T lymphocytes (CTL) population that can infiltrate, bind and kill tumor cells. In normal condition, there is an immune checkpoint regulation to balance immune response to minimize damage or autoimmune reaction. One of the most studied immune checkpoint molecules is programmed death-1 ligand (PD-L1). The interaction of PD-L1 molecule with its receptor, programmed death-1 receptor (PD-1) molecule, on CTL will inactivate CTL functions [18, 19]. High expression of PD-L1 has been observed in various types of cancers as well as in glioblastoma, suggesting potential clinical intervention using immune checkpoint inhibition [6, 20, 21]. In this paper we reported the analysis of patient overall survival (OS) in associations with PD-L1, IDH1 and MGMT status in anaplastic astrocytoma and glioblastoma patient receiving standard therapy. We also sought to understand better prognosis and to assess the efficacy of standard therapy.
The study was conducted in accordance with ethical approval from the Ethics Committee, Faculty of Medicine, Universitas Pelita Harapan/RS Siloam Lippo Village, Indonesia (No: FKUPH15022018).
Patient population: this retrospective study included 35 high grade glioma patients who underwent surgery at Siloam Karawaci Hospital, Jakarta from 2009 to 2015. The inclusion criteria for study were as follows: (i) Histologically diagnosed as high grade glioma (Anaplastic astrocytoma (AA) or Glioblastoma Multiforme (GBM)) according to WHO Classification; (ii) Patients were treated with Radiotherapy (RT) + Temozolomide (TMZ); (iii) Detailed clinical information at diagnosis and during follow up; (iv) Availability of tumor sample for molecular and immunohistochemistry (IHC) analysis. Any suspicious diagnosis for anaplastic oligodendroglioma was excluded from this population.
Sample preparation: formalin-fixed and parrafin-embbeded tissue blocks were processed for molecular and IHC analysis. Hematoxylin eosin (HE) staining were performed to examine the tumor area as well as to confirm the histological feature of sample by two experienced pathologists. For DNA extraction, the tumor area from 3 slides (of 4 μm thickness each) were carefully microdissected. The genomic DNA then extracted using QIAmp DNA Micro kit (Qiagen) according to the manufacturing protocol.
IDH1 mutation analysis: genotyping of IDH1 gene alteration on the mutation hotspot R132, was assesed using sanger sequencing method (Applied Biosystems 3500 Genetic Analyzer). Synthetic DNA was used as both mutant and wildtype controls (gBlocks Gene Fragment, Integrated DNA Technologies). The detailed methods and primers used for sequencing were referred to previous publications [22].
MGMT promotor methylation analysis: 20μL of samples genomic DNA (250 ng) of DNA was used for bisulphite modification process, using EZ DNA Methylation-Gold kit (Zymo Research). MYOD1 gene was used as positive control amplicon for bisulphite modification. The Methylation sensitive high resolution PCR approach was used to analyze the methylation status of MGMT promoter region, according to previous publication [23]. Cell line BT549 (ATCC HTB-122) and HCT-116 (ATCC CCL-247) were used as unmethylated control and partially methylated control respectively.
PD-L1 immunohistochemistry: we used anti-human PD-L1 antibody clone E1L3N®; from Cell Signalling Technology (USA), in ratio of 1:300 with overnight incubation time. Rabbit XP® was then used as secondary antibody followed by counterstaining with haematoxylin and slides dehydration. Expression of PD-L1 by cells was identified based on stained cell within the cell membrane and/or on the cytoplasm.
Statistical analysis: Kaplan-Meier method was used to draw the survival curves. Comparison of overall survival of cases with IDH1 wild type and R132H mutation; methylated and unmethylated MGMT promoter; and PD-L1 positive and negative was compared using the log-rank test. The statistical analyses were done using SPSS 16.0 (IBM Corporation, Armonk, NY, USA). The two-sided significance level was set at P<0.05.
Patients characteristics: Table 1, summarized the characteristics of the 35 patients in the study (66% males, 34% females); the median (± standard deviation) age was 51 ± 14.9 years old (range, 14 to 76 years old), under 50 years old was 17 (49%) while over 50 years old was 18 (51%). The high grade glioma in this study consists of anaplastic astrocytoma (AA) or grade III and glioblastoma (GBM) or grade IV. The AA group was 34% (12/35) and the GBM group was 66% (23/35) of patients. The molecular scoring including IDH1and MGMT showed that IDH1 wild type was 83% (29/35), IDH1 R132H mutation was 17% (6/35), MGMT methylation was 30% (7/23) and MGMT unmethylated was 70% (16/23). Immunohistochemistry scoring of PD-L1 negative expression was 88.6% (31/35) and PD-L1 positive was 11.4% (4/35) (Figure 1). The mean overall survival (OS) of all patients was 18.07±11.6 months. Sample size for all characteristics is n = 35. Sample size for MGMT methylation status was n = 23, where only patients with available MGMT data was included. *MGMT methylated patient numbers consisted of four patients with complete methylation and three with partial methylation.
Frequency of IDH1 mutation, MGMT promoter methylation and PD-L1 expression status: among a total 35 glioma cases, the frequency of IDH1, MGMT promoter methylation and PD-L1 expression status of glioma patients were represented in Figure 2. By molecular analysis, IDH1 R132H mutations were identified in five of twelve cases (42%) in the AA group, and one of twenty three cases (4.3%) in GBM group. Of a total thirty five cases, twelve cases were unable to be identified, thus only 23 cases were reported in this paper. MGMT promoter methylation was identified in four of eight cases (50%) in the AA group and three of twelve cases (20%) in the GBM. Of four MGMT methylated cases, three cases were partially methylated and were included in AA group. Tumor proportion score (TPS) value of PD-L1 expression showed two of twelve cases (17%) and two of twenty three cases (8.7%) were PD-L1 positive in AA and GBM groups, respectively. Of all cases, two cases were identified with both IDH1 R132H mutation and MGMT promotor methylation, and one of them was PD-L1 positive (< 10% TPS) (Table 2). Age of cases with both IDH1 R132H mutation and MGMT promotor methylation were over 50 years old and among AA group. Survival time for both cases were 42 and 32 months.
Overall survival of all glioma, AA and GBM cases based on IDH1 gene mutation: the overall survival based on IDH1 mutation status is summarized in Figure 3. Of a total 35 glioma patients, the estimated Kaplan-Meier survival with IDH1 wild type (IDH1 WT) was 13 months (range 11.25 to 14.75 months), while patients with IDH1 R132H mutation (IDH1 R132H) was 29 months (range 11.8 to 46.2 months). Survival in AA group with IDH1WT and IDH1 R132H were 27 months (range 15.3-38.7) and 29 months (range 11.8-46.2), respectively. For GBM group, the survival of patients with IDH1 WT was 11 months (range 7.9-14.1). The IDH1 R132H mutation in GBM group was found in only one patient with 14 months survival. In general, overall survival was better in cases with IDH1 R132H mutation in all glioma cases and both AA and GBM although the P values were not statistically significant (P > 0.05).
Overall survival of all glioma, AA and GBM cases based on MGMT methylation: median overall survival based on MGMT methylation was derived from cases with available MGMT methylation status data, which was 23 cases. Kaplan-Meier survival of MGMT met group in all glioma cases was 11 months (range 5.9 to 16.13 months) while for MGMT unmet group was 13 months (range 6.5 to 19.5 months) with no significant difference between groups (P > 0.05). Among the AA group, survival of cases with MGMT met was 27 months (range 3.5 to 50.5) while cases with MGMT unmet was 17 months (range 9.2 to 24.8). For GBM group, cases with MGMT met showed median survival at 11 months (7.8 to 14.2), while cases with MGMT unmet showed median survival at 8 months (range 4.6-11.4). Although MGMT methylation indicated a better survival in both AA and GBM cases when compared to the unmethylated groups, it was not statistically significant (Figure 4).
Overall survival of all glioma, AA and GBM cases based on PD-L1 expression: the expression of PD-L1 was measured by tumor proportion scoring or TPS. Overall survival of cases with PD-L1 negative was 13 months (range 10.7-15.3), while cases with PD-L1 positive was 12.75 months (range 0 to 42). Both AA and GBM groups showed better survival when PD-L1 expression was negative (estimate survival was 27 months within the range 17.9-36.1 months and 12 months within the range 9.3-14.7, respectively) when compared to PD-L1 positive group (estimate survival was 17 and 4.5 months, respectively), although the difference was not significant. Number of cases with PD-L1 positive in both AA and GBM groups (only 2 patients for each group) were relatively low in this study thus range of survival was not applicable (Figure 5).
Glioma is a malignant tumor with extremely poor prognosis. Currently, the main therapy for glioma is a comprehensive treatment including surgical resection, radiotherapy and chemotherapy [9, 23-26]. Although low grade glioma patients have better survival than patients with high grade (WHO grade III/IV) glioma, all low grade gliomas eventually progress to high grade glioma and death. Molecular markers such as IDH1 mutation, MGMT methylation and PD-L1 are among the most recent and widely studied tools for diagnosis and treatment guidance [22, 27]. In this study we performed analysis of overall survival for high grade glioma (anaplastic astrocytoma/grade III and glioblastoma / grade IV) that received same standard treatment (surgical resection, radiation and temozolomide) based on its prognostic marker; IDH1 mutation, MGMT methylation and PD-L1 expression.
Previous studies showed that IDH1 R132H mutation was present in 80% of WHO grade II and III gliomas but less than 5% of primary glioblastoma. Our result showed that 42% of AA group, while only 4.3% of GBM group, possessed IDH1 R132H mutation. High percentage of IDH1 mutation in AA group is consistent with the former finding that IDH gene 1 or 2 is mutated in the vast majority of WHO grade II or III gliomas [12, 28]. Therefore, a case of GBM with IDH1 mutation in our study suggest that the tumor has developed from a lower grade (secondary GBM). Both AA and GBM groups in our study with IDH1 mutation showed better survival when compared to IDH1 wild type group. This is probably because mutation in IDH1 gene may disrupt the function of IDH1 enzyme as the main catalyst for cellular metabolism, redox state and DNA repair in tumor cells, thus leading to the less progressive tumor and better survival of patients. On the other hand, as IDH 1 and 2 have roles in oncogenesis, their occurrence prompted the development of IDH1/2-mutant inhibitors such as Enasidenib [29, 30].
Methylation of MGMT promoter is a commonly observed change as molecular prognostic factor and also predicts a favourable response to alkylating agent chemotherapy [31-33]. On the basis of our data, MGMT promoter methylation is more frequent in AA cases when compard to GBM (50% and 20%, respectively). It appears that MGMT promoter methylation in both AA and GBM groups gives better survival when compared to those of without MGMT promoter methylation, points its important role as prognostic factor in glioma cases. Part of the aggressive nature of glioma is related to its ability to escape immune system surveillance by an overexpression of immune checkpoint molecules such as PD-L1. Thus, the status of PD-L1 expression is important for considering therapy combination with immune checkpoint molecule blockade drugs. The rate of PD-L1 positive cases in our study seems relatively low in both AA and GBM groups (17% and 8.7%, respectively) when compared with glioma and solid tumor types in other studies [20, 34]. In our present study, cases tested positive PD-L1 seems to have shorter survival of both AA and GBM cases. However, there are only 4 PD-L1 positive cases (2 for each groups) in this study which may not enough to represent overall glioma cases.
We also report 2 AA cases within the similar median age (both with age over 50 years), both possessed good prognostic markers ie positive IDH1 R132H mutation and MGMT promoter methylation. However, one case was with negative PD-L1 and the other was positive PD-L1. In consequence of the good prognostic markers, both cases seem to have relatively long survival rate (over two years), although case with PD-L1 positive appears to have shorter survival in comparison to that of with negative PD-L1 status (42 months and 32 months, respectively). Based on literatures, studies on the prognostic impact of tumoral PD-L1 expression have shown inconsistent results across tumor types [35-37]. In our series including a total of 35 glioma cases, here we provide evidence of high grade glioma case with positive IDH1 R132H mutation and MGMT promoter methylation that reached the long-term survival rate (over 36 month) when the PD-L1 expression status is negative, in comparison to its positive PD-L1 counterpart. In addition, the histological grade of glioma still the main indicator of patient survival, ie grade III glioma has longer survival than grade IV glioma, regardless to their molecular prognostic marker and PD-L1 status. Our study is limited by the sample size and our results surely need confirmation in larger cohort.
This study shows that PD-L1 is detectable in minority of glioma samples, suggesting that the immune checkpoint blockade therapy would be a benefit for those patients. Molecular markers such as IDH1 R132H mutation and MGMT methylation are relevant for good prognostic marker in our data. High expression of PD-L1 apparently does not indicate a worse overall survival when IDH1 R132 mutation and MGMT methylation are positive. The survival rate is likely associated with histological grade of the glioma. Taken together, standardized glioma treatment is still relevant for high grade glioma management in respect of overall survival analysis.
What is known about this topic
- Glioblastoma multiforme (GBM) constitutes a lethal diagnosis, with a median survival time of 14-16 months, while 36 months is considered as long-term survival;
- Standard therapy for GBM includes surgical resection, followed by radiation and co-administration of temozolomide (TMZ), an oral alkylating agent;
- Beside WHO grading system, clinical finding, neurologic performance, tumour location and molecular alterations, are used to predict the prognosis of patients.
What this study adds
- PD-L1 is detectable in minority of glioma samples, suggesting that the immune checkpoint blockade therapy would be a benefit for those patients; IDH1 R132H mutation and MGMT methylation are relevant for good prognostic marker;
- High expression of PD-L1 apparently does not indicate a worse overall survival when IDH1 R132 mutation and MGMT methylation are positive;
- Standardized glioma treatment is still relevant for high grade glioma management in respect of overall survival analysis.
The authors declare no competing interests.
Contributions: (I) Conception and design: J July; (II) Administrative support: J July; S Tandean, ML Loe; (III) Provision of study materials: J July; (IV) Collection and assembly of data: J July, D Patricia, PY Gunawan, Handrianto, TE Ginting, TP Putra, Z Wuisan, D Budhiarko, N Masykura, G Prayogi, AR Utomo; (V) Data analysis and interpretation: All authors; (VI) Manuscript writing: All authors; (VII) All authors read and approved the final version of this manuscript.
We would like to thank Nathaniel Ryan Sanjaya for an excellent technical assistance. Funding for this research had been provided in parts by Innogen Kalbiotech, and PT Kalbe Farma Tbk.
Table 1: characteristics of glioma patients
Table 2: PD-L1 status of cases with the presence of IDH1 R132H mutation and MGMT promoter methylation
Figure 1: characterization of PD-L1 expressions in gliomas. Gliomas grade III (Anaplastic Astrocytoma/AA panels A to D) and grade IV (Glioblastoma Multiforme/GBM panels E to H); hematoxylin
eosin images of AA (panels A and C) and GBM (panels E and G); representatives immunohistochemistry of PD-L1 negative AA (panel B) and GBM (panel F) and PD-L1 positive AA (panel D) and GBM (panel H)
Figure 2: proportion of IDH1 wild type (WT) and R132H mutation (A); MGMT promotor methylation (MGMT met) and unmethylated (MGMT unmet) (B); and PD-L1 negative and PD-L1 positive (C) in anaplastic astrocytoma (AA) and glioblastoma (GBM) patients
Figure 3: survival of glioma patients based on IDH1 mutation status in all cases (A), anaplastic astrocytoma (AA) (B) and glioblastoma (GBM) (C) cases. IDH1 WT = IDH1 wild type, IDH1 R132H = IDH1 mutation arginine to histidine at amino acid 132
Figure 4: survival of glioma patients based on MGMT methylation status in all cases (A), anaplastic astrocytoma (AA) (B) and glioblastoma (GBM) (C). MGMT met = MGMT methylation, MGMT unmet = MGMT unmethylated
Figure 5: survival of glioma patients based on PD-L1 expression in all cases (A), anaplastic astrocytoma (AA) (B) and glioblastoma (GBM) (C)
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016 Jun;131(6):803-20. PubMed | Google Scholar
- Komori T. The 2016 WHO Classification of Tumours of the Central Nervous System: The Major Points of Revision. Neurol Med Chir (Tokyo). 2017;57(7):301-311. PubMed | Google Scholar
- Stieber VW. Low-grade gliomas. Curr Treat Options Oncol. 2001;2(6):495-506. PubMed | Google Scholar
- Wesseling P, Capper D. WHO 2016 Classification of gliomas. Neuropathol Appl Neurobiol. 2018;44(2):139-150. PubMed | Google Scholar
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol (Berl). 2007;114(2):97-109. PubMed | Google Scholar
- Garber ST, Hashimoto Y, Weathers S-P, Xiu J, Gatalica Z, Verhaak RGW et al. Immune checkpoint blockade as a potential therapeutic target: surveying CNS malignancies. Neuro-Oncol. 2016;18(10):1357-136. PubMed | Google Scholar
- Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M et al. Long-term survival with glioblastoma multiforme. Brain J Neurol. 2007;130(Pt 10):2596-2606. PubMed | Google Scholar
- Davis ME. Glioblastoma: Overview of Disease and Treatment. Clin J Oncol Nurs. 2016;20(5 Suppl):S2-. PubMed | Google Scholar
- Paolillo M, Boselli C, Schinelli S. Glioblastoma under Siege: An Overview of Current Therapeutic Strategies. Brain Sci. 2018;8(1)15. PubMed | Google Scholar
- Loe ML, Maliawan S. Spontaneous recovery of Medial Prefrontal Syndrome following Giant Olfactory Groove Meningioma resection: A case report. Bali Med J. 2019;8(2):380. Google Scholar
- Stupp R, Brada M, van den Bent MJ, Tonn J-C, Pentheroudakis G, ESMO Guidelines Working Group. High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014 Sep;25 Suppl 3:iii93-101. PubMed
- Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765-773. PubMed | Google Scholar
- Pegg AE, Dolan ME, Moschel RC. Structure, function, and inhibition of O6-alkylguanine-DNA alkyltransferase. Prog Nucleic Acid Res Mol Biol. 1995;51:167-223. PubMed | Google Scholar
- Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343(19):1350-1354. PubMed | Google Scholar
- Smrdel U, Popovic M, Zwitter M, Bostjancic E, Zupan A, Kovac V et al. Long-term survival in glioblastoma: methyl guanine methyl transferase (MGMT) promoter methylation as independent favourable prognostic factor. Radiol Oncol. 2016;50(4):394-401. PubMed | Google Scholar
- Uno M, Oba-Shinjo SM, Camargo AA, Moura RP, Aguiar PH de, Cabrera HN et al. Correlation of MGMT promoter methylation status with gene and protein expression levels in glioblastoma. Clin Sao Paulo Braz. 2011;66(10):1747-1755. PubMed | Google Scholar
- Li Q, Guo J, Wang W, Wang D. Relationship between MGMT gene expression and treatment effectiveness and prognosis in glioma. Oncol Lett. 2017;14(1):229-233. PubMed | Google Scholar
- Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8(9):1069-1086. PubMed | Google Scholar
- Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117(5):1137-1146. PubMed | Google Scholar
- Berghoff AS, Kiesel B, Widhalm G, Rajky O, Ricken G, Wöhrer A et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro-Oncol. 2015;17(8):1064-1075. PubMed | Google Scholar
- Nduom EK, Wei J, Yaghi NK, Huang N, Kong L-Y, Gabrusiewicz K et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro-Oncol. 2016;18(2):195-205. PubMed | Google Scholar
- Heiland DH, Haaker G, Delev D, Mercas B, Masalha W, Heynckes S et al. Comprehensive analysis of PD-L1 expression in glioblastoma multiforme. Oncotarget. 2017;8(26):42214-42225. PubMed | Google Scholar
- Martínez-Garcia M, Álvarez-Linera J, Carrato C, Ley L, Luque R, Maldonado X et al. SEOM clinical guidelines for diagnosis and treatment of glioblastoma (2017). Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mex. 2018;20(1):22-28. PubMed | Google Scholar
- Suryadevara CM, Verla T, Sanchez-Perez L, Reap EA, Choi BD, Fecci PE et al. Immunotherapy for malignant glioma. Surg Neurol Int. 2015;6(Suppl 1):S68-77. PubMed | Google Scholar
- Chen Q, Li T, Yue W. Drug response to PD-1/PD-L1 blockade: based on biomarkers. OncoTargets Ther. 2018;11:4673-4683. PubMed | Google Scholar
- Carlsson SK, Brothers SP, Wahlestedt C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol Med. 2014;6(11):1359-1370. PubMed | Google Scholar
- Claus EB, Walsh KM, Wiencke JK, Molinaro AM, Wiemels JL, Schildkraut JM et al. Survival and low-grade glioma: the emergence of genetic information. Neurosurg Focus. 2015;38(1):E6. PubMed | Google Scholar
- Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol (Berl). 2008;116(6):597-602. PubMed | Google Scholar
- Popovici-Muller J, Saunders JO, Salituro FG, Travins JM, Yan S, Zhao F et al. Discovery of the First Potent Inhibitors of Mutant IDH1 That Lower Tumor 2-HG in Vivo. ACS Med Chem Lett. 2012;3(10):850-855. PubMed | Google Scholar
- Molenaar RJ, Maciejewski JP, Wilmink JW, van Noorden CJF. Wild-type and mutated IDH1/2 enzymes and therapy responses. Oncogene. 2018;37(15):1949-1960. PubMed | Google Scholar
- Weller M, Tabatabai G, Kästner B, Felsberg J, Steinbach JP, Wick A et al. MGMT Promoter Methylation Is a Strong Prognostic Biomarker for Benefit from Dose-Intensified Temozolomide Rechallenge in Progressive Glioblastoma: The DIRECTOR Trial. Clin Cancer Res Off J Am Assoc Cancer Res. 2015;21(9):2057-2064. PubMed | Google Scholar
- Brandes AA, Franceschi E, Ermani M, Tosoni A, Albani F, Depenni R et al. Pattern of care and effectiveness of treatment for glioblastoma patients in the real world: Results from a prospective population-based registry. Could survival differ in a high-volume center? Neuro-Oncol Pract. 2014;1(4):166-171. PubMed | Google Scholar
- Wang M, Dignam JJ, Won M, Curran W, Mehta M, Gilbert MR. Variation over time and interdependence between disease progression and death among patients with glioblastoma on RTOG 0525. Neuro-Oncol. 2015;17(7):999-1006. PubMed | Google Scholar
- Pan Y, Zheng D, Li Y, Cai X, Zheng Z, Jin Y et al. Unique distribution of programmed death ligand 1 (PD-L1) expression in East Asian non-small cell lung cancer. J Thorac Dis. 2017;9(8):2579-2586. PubMed | Google Scholar
- Li Y, Liang L, Dai W, Cai G, Xu Y, Li X et al. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol Cancer. 2016;15(1):55. PubMed | Google Scholar
- Hirai A, Yoneda K, Shimajiri S, Kuroda K, Hanagiri T, Fujino Y et al. Prognostic impact of programmed death-ligand 1 expression in correlation with human leukocyte antigen class I expression status in stage I adenocarcinoma of the lung. J Thorac Cardiovasc Surg. 2018;155(1):382-392.e1. PubMed | Google Scholar
- Takamori S, Takada K, Azuma K, Jogo Y, Kinoshita F, Kozuma Y et al. Prognostic Impact of PD-L2 Expression and Association with PD-L1 in Patients with Small-cell Lung Cancer. Anticancer Res. 2018;38(10):5903-5907. PubMed | Google Scholar
Search
This article authors
On Pubmed
On Google Scholar
Citation [Download]
Navigate this article
Similar articles in
Key words
Tables and figures
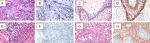 Figure 1: characterization of PD-L1 expressions in gliomas. Gliomas grade III (Anaplastic Astrocytoma/AA panels A to D) and grade IV (Glioblastoma Multiforme/GBM panels E to H); hematoxylin eosin images of AA (panels A and C) and GBM (panels E and G); representatives immunohistochemistry of PD-L1 negative AA (panel B) and GBM (panel F) and PD-L1 positive AA (panel D) and GBM (panel H)
Figure 1: characterization of PD-L1 expressions in gliomas. Gliomas grade III (Anaplastic Astrocytoma/AA panels A to D) and grade IV (Glioblastoma Multiforme/GBM panels E to H); hematoxylin eosin images of AA (panels A and C) and GBM (panels E and G); representatives immunohistochemistry of PD-L1 negative AA (panel B) and GBM (panel F) and PD-L1 positive AA (panel D) and GBM (panel H)















