The prevalence, risk factors and antifungal sensitivity pattern of oral candidiasis in HIV/AIDS patients in Kumba District Hospital, South West Region, Cameroon
Ngwa Fabrice Ambe, Njunda Anna Longdoh, Patience Tebid , Tanyi Pride Bobga, Claude Ngwayu Nkfusai, Sangwe Bertrand Ngwa, Frankline Sanyuy Nsai, Samuel Nambile Cumber
Corresponding author: Samuel Nambile Cumber, Faculty of Health Sciences, University of the Free State, Bloemfontein, South Africa 
Received: 18 Jan 2019 - Accepted: 02 Mar 2020 - Published: 19 May 2020
Domain: HIV epidemiology,Infectious diseases epidemiology,Infectious disease
Keywords: HIV/AIDS, oral candidiasis, CD4 cell, antifungal
©Ngwa Fabrice Ambe et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Ngwa Fabrice Ambe et al. The prevalence, risk factors and antifungal sensitivity pattern of oral candidiasis in HIV/AIDS patients in Kumba District Hospital, South West Region, Cameroon. Pan African Medical Journal. 2020;36:23. [doi: 10.11604/pamj.2020.36.23.18202]
Available online at: https://www.panafrican-med-journal.com//content/article/36/23/full
Research 
The prevalence, risk factors and antifungal sensitivity pattern of oral candidiasis in HIV/AIDS patients in Kumba District Hospital, South West Region, Cameroon
The prevalence, risk factors and antifungal sensitivity pattern of oral candidiasis in HIV/AIDS patients in Kumba District Hospital, South West Region, Cameroon
Ngwa Fabrice Ambe1, Njunda Anna Longdoh1, Patience Tebid1, Tanyi Pride Bobga1, Claude Ngwayu Nkfusai2,3, Sangwe Bertrand Ngwa1, Frankline Sanyuy Nsai2,3, Samuel Nambile Cumber4,5,6,&
1Department of Medical Laboratory Sciences, Faculty Health Sciences, University of Buea, Buea, Cameroon, 2Department of Microbiology and Parasitology, Faculty of Science, University of Buea, Buea, Cameroon, 3Cameroon Baptist Convention Health Service(CBCHS), Yaoundé, Cameroon, 4Office of the Dean, Faculty of Health Sciences, University of the Free State, Bloemfontein, South Africa, 5Centre for Health Systems Research & Development, University of the Free State, Bloemfontein, South Africa, 6School of Health Systems and Public Health, Faculty of Health Sciences, University of Pretoria, Pretoria, South Africa
&Corresponding author
Samuel Nambile Cumber, Faculty of Health Sciences, University of the Free State, Bloemfontein, South Africa
Introduction: oral candidiasis is one of the most common opportunistic infection in HIV/AIDS patient and it is caused by Candida species. The low absolute CD4+T-lymphocyte count has traditionally been cited as the greatest risk factor for the development of Oral Candidiasis. The aim of this study was to identify Candida species isolated from the oral cavity of HIV/AIDS patients, to determine their in vitro antifungal susceptibility and to investigate the possible risk factors associated with oral candidiasis.
Methods: this was a hospital based cross sectional study that was carried out for a period of 3 months amongst HIV/AIDS patients in Kumba District Hospital, whether on HAART or not. Mouth swabs were collected from 378 participants using sterile cotton wool swabs and 5ml venous blood were collected for determination of CD4 cell. Candida species were isolated and identified. Antifungal sensitivity testing was performed using modified kirby-bauer susceptibility testing technique.
Results: Candida species were present in 42.86% of the samples and Candida albicans was the most prevalent (60.2%) amongst the six Candida isolates identified, followed by Candida glabrata (16.9%), Candida krusei (12.3%), Candida tropicalis (6.4%), Candida parapsilosis (2.3%) and Candida pseudotropicalis (1.8%) .Pregnancy, oral hygiene and antibiotic usage were significantly associated with oral candidiasis in HIV/AIDS patients (P<0.05). Oral candidiasis was mostly frequent in HIV/AIDS patients between 21-40 years. A CD4 cell count less than 200 cells/μl was a significant risk factor for acquiring oral candidiasis in HIV/AIDS patients (P<0.001). Nystatin was the most sensitive drug (83.6%) meanwhile ketonazole was the most resistant drug (29.2%), followed by fluconazole (24.6%) to all oral Candida isolates.
Conclusion: oral Candida colonization occurs more frequently in HIV/AIDS patients and the is a need for the government to implement regular checks for opportunistic infections in HIV/AIDS patients, including oral candidiasis in HIV/AIDS patients to monitor disease progression and prevent subsequent complications such as candidemia and diarrhea.
The acquired immune deficiency syndrome (AIDS) is caused by the human immunodeficiency virus (HIV) and is characterised by progressive damage to the immune system, is the most important public health problem of the 20th century. HIV/AIDS continues to spread globally and remains a worldwide pandemic affecting about 36.7 million people [1]. It still continues to grow in countries of all incomes with a greater impact on the more vulnerable developing world and has emerged as a global crisis since its discovery in the summer of 1981[1, 2]. Even though the prevalence of HIV among adults is remarkably small (3.4%) [3] in Cameroon as compared with other sub-Sahara African countries like South Africa (19%) and Zambia (12.5%) [1, 3], it still remains a measure public health problem. Owing to a weakened immune system, the infected person is placed at an increased risk of a wide variety of opportunistic infections [4, 5]. Oral candidiasis (OC) is one of the most common fungal opportunistic infections in immunocompromised individuals [6]. Oral candidiasis occurs in up to 95% of human immunodeficiency virus (HIV)-infected individuals during the course of their illness [6, 7] and is a prognostic indicator for acquired immune deficiency syndrome (AIDS) [6, 7]. Worldwide, it is estimated that 9.5 million HIV patients suffer from oral candidiasis [6, 8]. Candida species normally colonise the gastrointestinal tract of healthy individuals and in HIV/AIDS patients. The infection is commonly acquired endogenously except in a few cases where strains can be transmitted from person to person [9].
Oral candidiasis is mainly caused by Candida albicans, which accounts for up to 81% of cases among HIV-infected individuals [6]. However, non-albicans Candida species have been implicated in colonization of the oral cavity, eventually causing infection in 20-40% of immunocompromised individuals [6]. Many factors have been suggested as risk factors for oral colonization; these include diabetes mellitus, head and neck cancer, smoking, the use of oral prostheses, age, race and poor nutritional status [10]. Others are reduced salivary flow, the use of antibiotics, immunosuppressive states (pregnancy) and even the presence of other locally available microflora [10]. In HIV-positive individuals, it has also been observed that the low CD4+ count, high HIV viral load, non-availability or non-usage of highly active antiretroviral therapy (HAART) and the type of antiretroviral (ARV) medication have been explored as possible factors that influence oral Candida carriage as well as with oral candidiasis in HIV patients [10, 11]. These data suggest that a decrease in oropharyngeal Candida carriage and oral candidiasis in HIV/AIDS patients can be achieved by initiating patients on highly active antiretroviral treatment (HAART) without the need for specific antifungal therapy [12]. Thus a prompt diagnosis of Candida infection in HIV patients and assessment of the immune status can have bearing on treatment of such infections and improve the general wellbeing of the PLWHA (people living with HIV/AIDS) [12]. The low absolute CD4+ T-lymphocyte count has traditionally been cited as the greatest risk factor for the development of oral candidiasis and current guidelines suggest increased risk once CD4+ T lymphocyte counts fall below 200 cells/μl [13]. Also, the increased incidence of mucosal and probably deep systemic forms of candidiasis has consequently made the use of antifungal agents the best option by clinicians so as to be able to control these pathogens [6, 14].
The widespread use of these antifungals has consequently led to an increase in antifungal resistance. Some authors have further observed a noticeable shift toward non albicans species with relative resistance to fluconazole and itraconazole [14]. The species spectrum and resistance of Candida isolates to currently available antifungal drugs is therefore a highly relevant factor because it causes important implications for morbidity and mortality [13, 14]. During the last few decades, the spectrum of infections has undergone a drastic change; organisms with minimal or no pathogenic role have emerged as potent pathogens and organisms once susceptible have become multidrug resistant as in the case of non albicans species [6, 13-15] and Up till now, no cure exists for HIV/AIDS. It then follows that controlling opportunistic pathogens associated to this pandemic remains one surer way of managing infected individuals who have the scourge [14-16]. The aim of this study was to identify Candida species isolated from the oral cavity of HIV/AIDS patients, to determine their in vitro antifungal susceptibility and to investigate the possible risk factors associated with the infection in the view to contributing to a better management of Candida infections in HIV/AIDS patients.
Study design: this study was a hospital based cross sectional study carried out between 6th April to 28th June 2018. At enrolment an informed consent and assent form was obtained and each study participant was asked to complete a questionnaire which consisted of socio-demographic and personal details, history of present illness, clinical signs and symptoms, and so forth. Participant on HAART were identified as with or without protease inhibitor.
Study area: this study was conducted in Kumba District Hospital HIV/AIDS treatment center which has about 3860 active HIV/AIDS patient on treatment, which is located in Meme Division, South West Region Cameroon.
Sample size: sample size was calculated from the Lorenz formula:
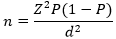
Where n is the sample size, Z is the confidence interval (95%) Z=1.95, P is the pre-estimated prevalence of 54.1% obtained from a study carried out by Njunda et al. in 2013 in Cameroon and d is the margin error (5%). The sample size calculated was 378 participants.
Study population: all HIV positive Patients of both sexes and all age groups whether on HAART or not were eligible for this study. Only those HIV seropositive subjects who have not received any specific antifungal therapy in the preceding three months were enrolled.
Ethical considerations: ethical review and clearance was soughed from the Institutional review board (IRB/FHS UB) and administrative clearance from the regional delegation of public health, the Kumba Health district and from the director of the Kumba District Hospital.
Sample collection: mouth swabs were collected from all participants using sterile cotton wool swabs and the swab was labeled with patient code. 5ml venous blood was collected for determination of CD4 cell.
Laboratory diagnosis
CD4 cell count: the blood specimen was used for determination of CD4 cell count using flow cytometry (pima counter) and the counts was categorized according to standards of the WHO, as severe when counts <200 cells/μl; low (200-349 cells/μl);moderate(350-499 cells/μl) and high when counts ≥500 cells/μl [17].
Microscopy: oropharyngeal swab samples were microscopically examined on a clean grease free glass slide using the 10X and 40Xobjectives for the presence of pus cells and of small, round to oval, thin-walled, clusters of budding yeast cells and branching pseudohyphae characteristically typical of Candida. Smears were made and Gram stained and latter observed at 100X for fungi elements [18].
Culture: oropharyngeal swab samples were inoculated on Sabouraud dextrose agar (SDA) impregnated with Chloramphenicol and incubated at 35°C(±2°C) for 24hours under aerobic conditions for the observation of colonies which were characteristically white to cream coloured, smooth, glabrous yeast like in appearance. Colonies were Gram stained and sub-cultured on chrom agar Candida media and SDA impregnated with Chloramphenicol for identification and antibiogram respectively [18].
Germ tube test: C.albicans can be identified presumptively by a simple germ tube test. Single colony was inoculated in human serum and incubated for 2-4 hours at 37°C and observed for germ tube formation under the microscope using the 40x objectives [18].
Chrom agar Candida medium: the CHROM agar Candida (Paris, France) is a selective and differential chromogenic medium that is useful for the identification of various Candida species. This medium is based on direct detection of specific enzymatic activities by adding multiple chemical dyes that is substrates of fluorochromes to media. Due to the chromogenic substrates added in the medium, the Candida colonies of various species produce different colours, thus allowing the direct identification of these Candida species on the isolation plate [19]. Discrete colony were pick from the sabouroud dextrose agar impregnated with chloramphenicol an emulsified in distilled water in a sterile test tube and inoculated on chrom agar Candida medium by streaking the entire surface of the test plate and incubated for 18-24 hours, after 24 hours colour change was observed which indicate a particular type of Candida species (Table 1) [19].
Antifungal sensitivitys testing: antifungal susceptibility test to fluconazole (25μg), itraconazole (10μg), ketoconazole (10μg), nystatin (100μg), miconazole (50μg), Econazole(50μg), and amphotericin B (20μg) were performed by modified kirby-bauer susceptibility testing technique as described by Clinical and Laboratory Standards Institute guidelines (CLSI guidelines) (Table 2) [20].
Statistical analysis: data were recorded on register forms and entered in a Microsoft Excel database in a secure computer and analysis was done with SPSS version 20 and EPI info version 7. Data were statistically described in terms of frequencies and percentages. The Chi-squared test was applied to analyse associations of Candida colonization with CD4 cell, different HAART regimen and oral hygiene. Multivariate analysis was applied to analyze risk factors associated with Candida colonization. A P value less than 0.05 was considered statistically significant at 95% confidence interval.
Demographic characteristics of HIV/AIDS patients in the Kumba District Hospital: 378 HIV/AIDS patients aged range from 3-72 years were involved in this study. The mean age of the participants was 40.30(SD=14.19), the median age was 40. Majority (47.1%) of the participants falls within the age group 20-40 years and age group ≤20 constituted 6.9%. more than half (276) of the participants were females. Majority (50.3%) of the participants ended their education in primary school, 38.6% in secondary school and 11.1% in the university. Most of the participants (61.1%) said that they wash their mouth once daily (Table 3).
Prevalence of oral candidiasis in HIV/AIDS patients: out of the 378 HIV/AIDS patients screened for oral candidiasis, 162 HIV/AIDS patient were positive for oral candidiasis given a prevalence of 42.86%, with female and male having a prevalence of 30.69% and 12.17% respectively.
Frequency of Candida species identified: in the total 162 samples that yielded growth of Candida species, single species were grown in 154 samples, mixed species in 8 samples and a total of 171 isolates were identified. Candida albicans had the highest frequency among all the species with a total number of 103(60.2%) compared to non albicans species. The non albicans species and their frequencies included Candida glabrata 29(16.9%), Candida krusei 21(12.3%), Candida tropicalis 11(6.4%), Candida parapsilosis 4(2.3%) and Candida pseudotropicalis 3(1.8%) which is the least species (Table 4). Among the 8 samples with multiple growth, Candida albicans was present in all the multiple growth. Candida albicans and Candida tropicalis were present in 3 samples, Candida albicans and Candida pseudotropicalis were present in 3 samples, Candida albicans and Candida tropicalis was present in a sample and Candida albicans and Candida krusei was present in a sample (Table 4).
Antifungal susceptibility pattern of oral Candida isolates: antifungal susceptibility pattern of 171 isolates of Candida species was determined (Table 5). Nystatin (83.6%) was the most sensitive drug, followed by amphotericin (76%) and miconazole (74.3%). The most resistant antifungal drugs were ketoconazole (29.2%), followed by fluconazole (24.4%). In our finding amongst, the six Candida species isolates, Candida albicans was the most sensitive Candida species to all the seven antifungal tested as compared to C. non-albicans, of the C. non-albicans species isolated Candida krusei and Candida tropicalis were the most resistant species to all the antifungal tested. We also observed resistance to fluconazole in 24.6% of all oral Candida isolates (Table 5).
Distribution of the prevalence of oral candidiasis according to sex, age and CD4 levels: the frequency of oral candidiasis was more among females (30.69%) than in males (12.17%) in this study (Table 6). However the association between gender and the prevalence of oral candidiasis was not statistically significant (P=0.593). The frequency of oral candidiasis was highest among individuals in the aged group 21 -40 years (Table 6). However the was no significant association between infection rate with age (P=0.755). Oral candidiasis was observe more frequently among individuals with CD4 cell less than 200 cells/μl (13.23%) (Table 6) However, the association between CD4 cell and the prevalence of oral candidiasis was statistically significant (P<0.001) (Table 6).
Association between the prevalence of oral candidiasis and protease inhibitor: the prevalence of oral candidiasis was lower (7.67%) in patients under HAART regimen with protease inhibitor than in patients under HAART regimen without protease inhibitor (35.19%) but these difference was not statistically significant (P=0.302) (Figure 1).
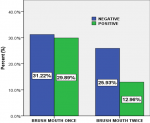
Association between the prevalence of oral candidiasis and oral hygiene: a low prevalence (12.96%) of oral candidiasis was noticed in patients who brush mouth twice daily and high prevalence (29.89%) of oral candidiasis in patients who brush mouth once daily. The Pearson chi square (χ2) test suggested that, the was a statistical significant difference between oral candidiasis and oral hygiene, (χ2(1)=8.909, P=0.003 (Figure 2).
Risk factors associated to oral candidiasis in HIV/AIDS patients in Kumba District Hospital: in the Bivariate analysis, brush mouth daily (oral hygiene) and CD4 cells/μl were significantly associated with oral candidiasis in HIV/AIDS patients. However, all variables with p-value <2 were taken to the multivariate analysis. After adjusting for confounders in the multivariate analysis, oral hygiene, pregnancy, antibiotic usage and CD4 level of the HIV/AIDS patients were significantly associated with oral candidiasis in HIV/AIDS patients (Table 7). The Odds of having oral candidiasis were significantly higher in HIV/AIDS patients who bush mouth once daily (AOR: 2.15; 95% CI: 1.32-3.50, p=0.002) compared to patients who brush mouth twice daily. Also, the Odds of having oral candidiasis were significantly higher in those that were pregnant (AOR: 2.04; 95%CI: 1.10-3.82; P=0.025) compared to those not pregnant (Table 7).
Oral candidiasis (OC) is one of the most common fungal opportunistic infections in immunocompromised individuals [6]. Oral candidiasis occurs in up to 95% of human immunodeficiency virus (HIV)-infected individuals during the course of their illness [6], and is a prognostic indicator for acquired immune deficiency syndrome (AIDS) [6]. Our results showed that 42.86% oral swabs collected from the oral cavity of HIV/AIDS patients, grew Candida colonies. This result is in line with reports from similar studies in Brazil [21, 22], where prevalence of 41.87% and 42% were recorded respectively. This prevalence (42.86%) recorded in this study is lower than that reported by Njunda et al. [15] and Nweze et al. [14], where prevalence of 54.1% and 60% were recorded respectively, these difference could probably be due to the fact that not all participants in these studies were on HAART and the advent of HAART has allowed for the suppression of viral replication to very low levels and a partial recovery of CD4 cells in patients with HIV, which has consequently reduced opportunistic infections.[11, 21, 22].
Candida albicans was the most frequent species isolated in these study which is consistent with previous studies [6, 15, 23], and this result contrasts with the observation in some countries in the Gulf region [24]. 60.2% of Candida albicans was isolated in this study which is in line with studies reported in Brazil [22, 23]. The possibly reason why Candida albicans constitute majority of the Candida species isolated from the oral cavity(60.2%) is probably facilitated by a number of virulence factors, the most important of which are the possession of multiple adhesins for adherence to host tissues, biofilm formation and secretion of hydrolytic enzymes proteases, phospholipases and haemolysins [25]. Also C. albicans reversibly converts from unicellular yeast cells to either pseudohyphal or hyphal growth, a morphogenesis phenomenon. The growth of hyphae, plays an important function in tissue invasion and resistance to phagocytosis [25]. Non albicans species accounted for about 39.8 of Candida species from the oral cavity. This result is in conformity to 44% reported in Brazil [23] and 20-40% reported in sub Saharan Africa [6]. Among these, Candida glabrata 29(16.9%) was the most frequent, which was followed by Candida krusei 22(12.3%), Candida tropicalis 11(6.4%), Candida parapsilosis 4(2.3%) and Candida psudotropicalis 3(1.8%), is a relatively common finding with other similar studies [6, 14, 23], but there were some striking differences in the species spectrum and percentage of recovered isolates. Candida glabrata, Candida krusei and Candida tropicalis were the most frequent non albicans species isolated from the HIV/AIDS patients in these study which is consistent with a study in sub Saharan Africa [6]. This is of public health concern because some of these non albicans species are intrinsic resistant to fluconazole which is the most commonly used azoles in developing countries [26].
Treatment against candidiasis varies substantially depending on the anatomical localization of the infection, the immune status of the patient, and the isolated species [27]. In order to understand the therapeutic differences in the isolates involved in oral candidiasis study population, we tested the sensitivity of these isolates. Our finding indicated that amongst all Candida isolates tested, nystatin was the most sensitive antifungal drug (83.6%), followed by amphotericin (76%) and miconazole (74.3%). This finding partly agree with a similar study carried out in Mexico [28], where nystatin and amphotericin were the most sensitive antifungal tested. This finding is in contrast with a similar study carried out in Cameroon by Njunda et al. [15], where ketonazole was found to be the most sensitive antifungal drug (85.5%). This disparity in result is probably due to decrease sensitivity of the Candida species isolated to azoles. Although in general Candida species are susceptible to both azoles and polyenes, antifungal susceptibility varies significantly between C. albicans and C. non-albicans. In our finding amongst, the six Candida species isolates, Candida albicans was the most sensitive Candida species to all the seven antifungal tested as compared to C. non-albicans. This is probably due to the fact that C. non-albicans are intrinsically resistant to azoles (fluconazole) [26]. Of the C. non-albicans species isolated Candida krusei and Candida tropicalis were the most resistant species to all the antifungal tested.
We observed resistance to fluconazole in 24.6% of oral Candida isolates; this prevalence is higher than the rates obtained in different areas in Cameroon [15] and Nigeria [29] but is in consistent with the 24.0% prevalence recorded in Benin City in Nigeria [30]. The possible explanations for the difference in rates include methodological, temporal, drug usage, immune status, regional variations and intrinsic resistance of the Candida species [14]. In Africa, fluconazole is usually considered to be the drug of choice in both the treatment and prophylactic prevention of fungal infections in HIV-infected individuals and people with HIV/AIDS [6]. This is due to its favourable pharmacokinetic profile, low toxicity, and availability. Consequently, increase resistance to the drug poses a threat not only to the treatment of oral candidiasis HIV/AIDS patient, but also to the management of systemic fungi infections HIV/AIDS patients. The other azoles recorded resistance which was closely similar to fluconazole; this is in contrary to previous study [28]. This is probably due to cross resistance to fluconazole, since all azoles exert the same mechanism of action and fluconazole was routinely administered to HIV/AIDS patients with clinical symptoms of candidiasis without prior antifungal susceptibility testing in these center.
The carriage rate was found to be more in women (30.69%) than in men (12.17%), but these different in prevalence was not statistically significant (P=0.598). This difference in prevalence is in conformity with studies carried out in Cameroon [15] and Mexico [28]. This difference is probably because most men rarely go for routine checkups until the disease has reached symptomatic stage and as at the time of this study only a few men consented. Participants around the age group of 21-40 year accounted for the highest prevalence rate (21.43%), but the difference in prevalence rate among age groups was not observed to be statistically significant (P=0.755). This result is in line with a study carryout in Cameroon [15], but contrary to study carried out in Mexico [28] which shows that Candida colonization was age dependent. This highest prevalence rate between these age group (21-40 year) is probably due to the fact that, they represent majority of the study participants and which constituted the highest active sex group which may also spread oral candidiasis exogenously.
The distribution of the prevalence of oral candidiasis at different CD4 cell levels, oral candidiasis (13.23%) was more frequent in individuals with <200 cells/μl and was a common observation with other studies [16, 21, 31]. This was a statistically significant association between the prevalence of oral candidiasis and low CD4 cells (P<0.001). This difference may be related to the decrease of the immunodeficiency of the immune system. This is supported by Fidel (2006), who suggests that Candida specific T cells do not become defective with immunosuppression, but a threshold number of CD4 cells are required to protect the oral cavity against infection by this normal commensal [32]. The prevalence of oral candidiasis was lower (7.67%) in patients under HAART combination with protease inhibitor than in patients under HAART combination without protease inhibitor (35.19%) which is conformity with a study carried out in Nigeria, [33] but these difference in prevalence of oral candidiasis was not statistically significant (P=0.302) and may be due to the fact that protease inhibitor group of ARV drugs exerts an early immune reconstitution-independent beneficial effect against oral candidiasis, which is entirely due to the protease inhibitor capacity to inhibit Sap enzymes of Candida secretory aspartyl proteinase (Sap) family that belongs to the same family as HIV-proteinase [34].
The was a statistical significant difference between the prevalence of oral candidiasis and oral hygiene (χ2(1)=8.909, P=0.003). A low prevalence (12.96%) of oral candidiasis was noticed in patients who brush mouth twice daily and a high prevalence (29.89%) of oral candidiasis in patients who brush mouth once daily. This association between oral candidiasis and oral hygiene was not consistent in a study carried out in Nigeria [33]. This difference in prevalence is probably due to that fact that good oral hygiene; however, is an essential part of treating oral thrush. Healthy adults and children can recover fairly easily from the infection, especially if they follow a complete oral care routine of twice-daily tooth brushing and daily proper flossing. In the Bivariate analysis, brush mouth daily (oral hygiene) and CD4 cells/μl were significantly associated with oral candidiasis in HIV/AIDS patients. However, all variables with p-value <2 were taken to the multivariate analysis. After adjusting for confounders in the multivariate analysis, oral hygiene, pregnancy, antibiotic used and CD4 level of the patient were significantly associated with oral candidiasis in HIV/AIDS patients. The Odds of having oral candidiasis were significantly higher in those that were pregnant (AOR: 2.04; 95%CI: 1.10-3.82; P=0.025) compared to those not pregnant and this finding is similar to previous studies [35, 36]. This is possibly due to the fact that pregnancy lead to immune suppression, altered bacteria flora and causes hormonal in balance which are major risk factor to oral candidiasis [35, 36]. The odds of Candida colonization were significantly greater in patients taking antibiotics in our multivariate analysis and this is consistent with observations of other investigators [32, 35]. This is probably due to the fact that broad spectrum antibiotics (Bactrim) alter the local oral flora creating a suitable environment for Candida to proliferate, which was usually administered to these patients in this center.
We concluded that, oral Candida colonization occurs more frequently in HIV/AIDS patients and are significantly more common in patients with CD4+ cell counts <200 cell/μl, thus placing them at higher risk of invasive infections caused by these pathogens. In our study a prevalence of 42.86% of oral candidiasis was obtained among HIV/AIDS patients, this prevalence among HIV/AIDS patient was not observed to be gender and age dependent in these study. A total of six Candida species were identified in this study, with Candida albicans (60.2%) been the most predominant Candida species identified. Polyenes (amphotericin and nystatin) recorded the highest sensitivity rate to all Candida species isolates. Azoles recorded almost similar sensitivity and resistance but ketonazole was the most resistant to all Candida isolates, followed by fluconazole and this is probably due to cross resistance to fluconazole. Candida albicans was the most sensitive Candida species isolate to all the seven antifungal tested while Candida krusei, Candida tropicalis and Candida glabrata were the most resistant species to all the seven antifungal tested. There was a statistically significant relationship between CD4 cell counts, oral hygiene, antibiotic usage, pregnancy and the prevalence of oral candidiasis in HIV/AIDS patients in this study.
What is known about this topic
- The is increase prevalence of oral candidiasis in HIV/AIDS;
- Low CD4 count has been seen as a major risk factors for oral candidiasis.
What this study adds
- Shows that the is increase resistance of Candida species to antifungals;
- Shows that poor oral hygiene and HAART without protease inhibitors remain a major risk factors for oral candidiasis in HIV/AIDS patients;
- Prevalence of Candidiasis in the South West Region, Cameroon.
The authors declare no competing interests.
NFA, NAL, TP, TPB, NCN, SBN, FSN and SNC conceived and designed the study: NFA implement the study: NAL and TP supervised the study: NFA conducted data analysis: NFA, NAL, TP, TPB, NCN, SBN, FSN and SNC interpreted study results: NFA and NAL wrote the first draft of the manuscript, NCN and SNC reviewed and corrected the manuscript. All authors approved the final copy.
We are grateful to all who participated in this research.
Table 1: identification of candida species using chrom agar medium
Table 2: zone diameter interpretation for fungi in milimeter
Table 3: the demographic characteristics of HIV/AIDS patients in Kumba District Hospital
Table 4: the distribution of different candida species isolated
Table 5: antifungal susceptibility profile of oral candida isolates
Table 6: distribution of the prevalence oral candidiasis according to gender, age and CD4 levels
Table 7: risk factors associated to oral candidiasis in HIV/AIDS patients
Figure 1: the proportion of those with oral candidiasis without protease inhibitor and those with protease inhibitor (PI)
Figure 2: the prevalence of oral candidiasis with regard to oral hygiene
- UNAIDS. The global HIV/AIDS EPIDEMIC. 2017.
- Shetti A, Gupta I, Charantimath SM. Oral candidiasis: aiding in the diagnosis of HIV a case report. Case Rep Dent. 2011;2011:929616. PubMed | Google Scholar
- Mundi C. Cameroon HIV/AIDS adult prevalence rate. 2017. 213-220.
- Damtie D1, Yismaw G, Woldeyohannes D, Anagaw B. Common opportunistic infections and their CD4 cell correlates among HIV-infected patients attending at antiretroviral therapy clinic of Gondar University Hospital, Northwest Ethiopia. BMC Res Notes. 2013 Dec 14;6:534. PubMed | Google Scholar
- Shahapur PR, Bidri BC. Recent trends in the spectrum of opportunistic infections in human immunodeficiency virus infected individuals on antiretroviral therapy in South India. J Nat Sci Biol Med. 2014 Jul;5(2):392-6. PubMed | Google Scholar
- Martha F Mushi, Oliver Bader, Liliane Taverne-Ghadwal, Christine Bii, Uwe Groß, Stephen E Mshanaa. Oral candidiasis among African human immunodeficiency virus-infected individuals:10years of systematic review and meta-analysis from sub-Saharan Africa. J Oral Microbiol. 2017; 9(1): 1317579. PubMed | Google Scholar
- Thanyasrisung P, Kesakomol P, Pipattanagovit P, Youngnak-Piboonratanakit P, Pitiphat W, Matangkasombut O. Oral Candida carriage and immune status in Thai human immunodeficiency virus-infected individuals. J Med Microbiol. 2014 May;63(Pt 5):753-759. PubMed | Google Scholar
- The Fungal Research Trust. How common are fungal diseases? Fungal Research Trust 20th Anniversary meeting. London June 18th 2011.
- Chander J. A textbook of Medical Mycology, 2nded. 2017:212-227. Google Scholar
- Gottfredsson M, Cox GM, Indridason OS, De Almeida GWD, Heald AE, Perfect JR. Association of plasma levels of human immunodefciency virus type 1 RNA and oropharyngeal Candida colonization. J Infect Dis. 1999;180(2):534-537. PubMed | Google Scholar
- Liu X, Liu H, Guo Z, Luan W. Association of asymptomatic oral candidal carriage, oral candidiasis and CD4+ lymphocyte count in HIV-positive patients in China. Oral Dis. 2006 Jan;12(1):41-4. PubMed | Google Scholar
- Maheshwari M, Kaur R, Chadha S. Candida Species Prevalence Profile in HIV Seropositive Patients from a Major Tertiary Care Hospital in New Delhi, India. J Pathog. 2016;2016:6204804. PubMed | Google Scholar
- Dessie MJ, Kechia AF, Yves SI, Aude DN, Claude N, Jean PD. Urinary tract candidiasis in HIV+ patients and sensitivity patterns of recovered Candida species to antifungal drugs in Dschang District Hospital (Cameroon). Int J Biol Chem Sci. 2017;11(3):1029-1038. Google Scholar
- Nweze EI, Ogbonnaya UL. Oral Candida isolates among HIV-infected subjects in Nigeria. J Microbiol Immunol Infect. 2011 Jun;44(3):172-7. PubMed | Google Scholar
- Longdoh AN, Jules CNA,Shey DN,Henri FK, Ejong CN,Tebit EK. Oral and Urinary Colonisation of Candida Species in HIV/AIDS Patients in Cameroon. Basic Sci Med. 2013;2(1):1-8. Google Scholar
- Vázquez-González D, Perusquía-Ortiz AM, Hundeiker M, Bonifaz A. Opportunistic yeast infections: candidiasis, cryptococcosis, trichosporonosis and geotrichosis. J Dtsch Dermatol Ges. 2013 May;11(5):381-93. PubMed | Google Scholar
- WHO, Laboratory guidelines for enumerating CD4 T lymphocytes in the context of HIV/AIDS, WHO, Geneva, Switzerland, 2007, Pp 16.
- Cheesbrough M. District Laboratory Practice in Tropical Countries. 2006;234-247. Google Scholar
- Beighton D, Ludford R, Clark DT, Brailsford SR, Pankhurst CL, Tinsley GF et al. Use of CHROM agar Candida medium for isolation of yeasts from dental samples. J Clin Micro. 1995;33(11):3025-3027. PubMed | Google Scholar
- Clinical and Laboratory Standards Institute. Zone Diameter Interpretive Standards, Corresponding Minimal Inhibitory Concentration(MIC) Interpretive Break points,and Quality Control Limits for Antifungal Disk Diffusion Susceptibility Testing of Yeasts:Informational Supplement. CLSI DocumentM44-S3.3rded. Villanova, PA:Clinical and Laboratory Standards Institute;2009. p. M44-S3.
- Rubeiro Rubeiro A, Tatiany OAM, Sérgio de A, Sílvio AFM, Silvia HM, Antonio Carlos RV. Oral carriage of Candida species in HIV-infected patients during highly active antiretroviral therapy(HAART) in Belém, Brazil. Oral medicine. July 2015;120:1. Google Scholar
- Junquira CJ, Simone FG, Rodnei DR, Júnia OB, Anna Carolina BP, Vanessa MCR. Oral colonization by yeasts in HIV-Positive patients in Brazil. Rev Inst Med Trop. January-February 2012;54(1):17-24. Google Scholar
- Ana LGT, Sirlei GM, Eduardo BM, Márcia BA, Conceição MP, Walter L et al. Antifungal drug susceptibility of candida isolated from HIV-Positive patients recruited at a Public Health Hospital Sao Luis, Moranhao, Brazil. Front microbio. 2017;8:298. PubMed | Google Scholar
- Mokaddas EM, Al-Sweih NA, Khan ZU. Species distribution and antifungal susceptibility of Candida blood stream isolates in Kuwait:a 10-year study. J Med Microbiol. 2007 Feb;56(Pt 2):255-259. PubMed | Google Scholar
- Sardi JCO, Scorzoni LT, Bernardi AM, Fusco-Almeida, Mendes Giannini MJS. Candida species:current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol. 2013 Jan;62(Pt 1):10-24. PubMed | Google Scholar
- Mushi MF, Mtemisika CI, Bader O, Bii C, Mirambo MM, Groß U et al. High Oral Carriage of Non-albicans Candida spp. among HIV-infected individuals. Int J Infect Dis. 2016 Aug;49:185-8. PubMed | Google Scholar
- Rex JH, Walsh TJ, Sobel JD, Filler SG, Pappas PG, Dismukes WE et al. Practice guidelines for the treatment of candidiasis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30(4):662-678. PubMed | Google Scholar
- Isadora C, Olga A, Callejas-N,Elva T, Arechiga-Ca, Rosa R. Mourino-P .Candida species diversity and antifungal susceptibility patterns in oral samples of HIV/AIDS patients in Baja California, Mexico. Med Mycol. 2017 Apr 1;55(3):285-294. PubMed | Google Scholar
- Okonkwo EC, Alo MN, Nworie O, Orji JO, Agah MV. Prevalence of oral Candida albicans infections in HIV seropositive patients in Abakaliki. Am J Life Sci. 2013;1(2):72-76 . Google Scholar
- Osaigbovo II, Lofor PV, Oladele RO. Fluconazole Resistance among Oral Candida Isolates from People Living with HIV/AIDS in a Nigerian Tertiary Hospital. J Fungi (Basel). 2017 Dec 8;3(4). pii: E69. PubMed | Google Scholar
- Antoine Berberi, Ziad Noujeim, Georges Aoun. Epidemiology of Oropharyngeal Candidiasis in Human Immunodefciency Virus/Acquired Immune Defciency Syndrome Patients and CD4+ Counts. J Int Oral Health. 2015 Mar;7(3):20-23. PubMed | Google Scholar
- Fidel PL. Candida-host interactions in HIV disease:relationships in oropharyngeal candidiasis. Adv Dent Res. 2006 Apr 1;19(1):80-4. PubMed | Google Scholar
- Owotade FJ, Patel M, Ralephenya TRMD, Vergotine G. Oral Candida colonization in HIV-positive women:associated factors and changes following antiretroviral therapy. J Med Microbiol. 2013 Jan;62(Pt 1):126-132. PubMed | Google Scholar
- De Bernardis F, Tacconelli E, Mondello F, Cataldo A, Arancia S, Cauda R et al. Anti-retroviral therapy with protease inhibitors decreases virulence enzyme expression in vivo by Candida albicans without selection of avirulent fungus strains or decreasing their anti-mycotic susceptibility. FEMS Immunol Med Microbiol. 2004 May 1;41(1):27-34. PubMed | Google Scholar
- Tsaim PW, Chen YT, Hsu PC, Lan CY. Study of Candida albicans and its interactions with the host:a mini review. Bio Med. 2013;3(1):51-64. Google Scholar
- Shafi FT, Padmaraj SR, Mullessery NP. Species distribution and antifungal susceptibility pattern of Candida causing oral candidiasis among hospitalized patients. Arch Med Health Sci. 2015;3:247-51. Google Scholar