The epidemiological characterization and geographic distribution of Hepatitis D virus infection in Libya
Mohamed Ali Daw, Amina Mohamed Daw, Nadia Emhemed Mohamed Sifennasr, Aisha Draha, Ahmed Daw, Ali Daw, Mohamed Ahmed, Ebtisam Mokhtar, Abdallah El-Bouzedi, Ibrahem Daw, Samia Adam, Saed Warrag
Corresponding author: Mohamed Ali Daw, Department of Medical Microbiology, Immunology, Faculty of Medicine, University of Tripoli, CC 82668, Tripoli, Libya 
Received: 12 Aug 2019 - Accepted: 03 Mar 2020 - Published: 14 Apr 2020
Domain: Epidemiology,Infectious diseases epidemiology
Keywords: Libya, hepatitis D virus, risk factors
©Mohamed Ali Daw et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Mohamed Ali Daw et al. The epidemiological characterization and geographic distribution of Hepatitis D virus infection in Libya. Pan African Medical Journal. 2020;35:120. [doi: 10.11604/pamj.2020.35.120.20055]
Available online at: https://www.panafrican-med-journal.com//content/article/35/120/full
Research 
The epidemiological characterization and geographic distribution of Hepatitis D virus infection in Libya
The epidemiological characterization and geographic distribution of Hepatitis D virus infection in Libya
Mohamed Ali Daw1,&, Amina Mohamed Daw2, Nadia Emhemed Mohamed Sifennasr1, Aisha Draha3, Ahmed Daw4, Ali Daw4, Mohamed Ahmed5, Ebtisam Mokhtar1, Abdallah El-Bouzedi6, Ibrahem Daw7, Samia Adam1, Saed Warrag8
1Department of Medical Microbiology et d'Immunology, Faculty of Medicine, University of Tripoli, CC 82668, Tripoli, Libya, 2Department of General Medicine, Faculty of Medicine, University of Tripoli, CC 82668, Tripoli, Libya, 3Department of Pharmacology, Faculty of Medicine, University of Tripoli, CC 82668, Tripoli, Libya, 4Tripoli Medical Centre, Faculty of Medicine, Tripoli, CC 82668, Tripoli, Libya, 5Department of Microbiology & Parasitology, Faculty of Veterinary Medicine, University of Tripoli, CC 82668, Tripoli, Libya, 6Department of Laboratory Medicine, Faculty of Biotechnology, Tripoli University, CC 82668, Tripoli, Libya, 7Department of Electric Engineering, Faculty of Engineering, University of Tripoli, CC 82668Libya, 8Department of Laboratory Medicine, Faculty of Biotechnology, Aljabel-Agarbi University, Nalot, Libya
&Corresponding author
Mohamed Ali Daw, Department of Medical Microbiology, Immunology, Faculty of Medicine, University of Tripoli,
CC 82668, Tripoli, Libya
Introduction: north Africa is known to be endemic for hepatitis D virus. However, data one the prevalence of this virus in Libya are scanty. This study aimed to determine the prevalence of hepatitis D virus infection in Libya and analyze the demographic factors associated with the infection, and also to assess the variations across the regions and districts.
Methods: a total of 1873 samples collected from all over the country were tested for antibodies against hepatitis B surface antigen and the results were correlated with demographic and geographic variables.
Results: the overall prevalence of hepatitis D virus infection was 1.7%. The prevalence rate was significantly high among those aged over 40 years (P < 0.001) and it was associated with intravenous drug use and coinfection with human immunodeficiency virus and/or hepatitis C virus infection (P < 0.001). The prevalence rates varied with geographic location and differed markedly within the regions the country. The highest rate reported was in the central region of Libya, followed by the western and eastern regions.
Conclusion: hepatitis D virus infection rate in Libya is considered to be low but is of some concern in some districts. This has been propagated by population displacement and African immigrants, indicating that a continuous epidemiological surveillance program should be implemented.
Hepatitis D virus (HDV) is an RNA viroid that infects only people in the presence of hepatitis B virus (HBV), which expresses hepatitis B envelope protein. HDV is responsible for the most severe form of acute and chronic viral hepatitis [1-4]. Worldwide, it is estimated that 2-8% of chronic HBV carriers are co-infected with HDV, corresponding to 10-20 million patients. Furthermore, HDV is transmitted by the same routes as HBV, including blood transfusion, sexual contact, intravenous drug abuse and parenteral transmission [5-7]. In the early 1980s, surveys showed that HDV was endemic worldwide but its prevalence rates are geographically diverse [8,9]. High prevalence rates of HDV have been reported in North African countries [10]. A recent meta-analysis showed that HDV is found in 5% of the general population in 20.7% of liver disease patients. The highest rates were reported in Egypt, Sudan and Mauritania, followed by Tunisia, Morocco, Algeria and Libya. The findings of that study should be interpreted with caution due to the small number of individuals tested, their demographic heterogeneity, and the quality of the testing methods used [11,12]. Libya has been plagued by a civil war since 2011, which has caused high rates of mortality, injury, population displacement, and major damage to the health care system at the structural and organizational levels. In addition, the breakdown of the security situation led to a heavy influx of African immigrants [13,14]. Together, these circumstances have maintained high levels of viral hepatitis transmission through injuries, blood and sexual contacts, and even unsafe blood transfusion [15]. Data on the impact of HDV in the country is even scarcer particularly as screening for anti HDV is not routine in HBsAg-positive patients. Therefore, it becomes important to reappraise the level of HDV infection among Libyan patients in order to plan a more effective preventive strategy. Hence, the objectives of this study were to determine the prevalence of HDV infection among Libyan patients, analyze the potential risk and demographic factors associated with the infection, and investigate the geographic variation of HDV within the different regions and districts of the country.
Study population: the study involved 1873 HBsAg-positive serum samples that were collected from all Libyan regions and districts between 2015 and 2018. Appendix illustrates the geographic locations, regions, districts and population density. The national screening policy for HBV in Libya, which is uniform all over the country, includes screening of blood donors, preoperative patients, pregnant women and kidney and liver disease patients, as well as a mandatory pre-marriage screening. The study population included only those who were confirmed to be HBsAg-positive in the public health care system in Libya between 2015 and 2018, who were identified from the state wide data base with clear demographic and epidemiological information [11,12]. The data collected included region of origin, gender, age, level of education, marital status, and other related risk and demographic factors as previously described (12879_2013_2969_MOESM1_ESM.doc )[11].
Laboratory diagnosis: the study included all the samples that were HBsAg-positive and had detectable HBV DNA according to preliminary screening using “Real Time HBV Viral Load Assay” (Abbott Laboratories, IL). Anti-HDV antibodies in EDTA plasma samples were detected by using an enzyme-linked immunosorbent assay (ETI-AB-DELTAK-2, Diasorin, Saluggia, Italy) [11].
Statistical analysis: data were coded and entered into a database, which was then cleaned and verified [12]. Demographic and risk factors were compared between patients using chi-square tests or Fisher's exact test for categorical variables and Mann-Whitney U tests for continuous variables. Variables were considered statistically significant in the bivariate analysis when P < 0.001. All calculations were undertaken by using IBM SPSS Statistics for Windows, version 20.0 (IBM Corp., Armonk, NY).
Ethics approval and consent to participate: the study was approved by the Libyan National Ethical Committee (Approval No. LY NS, HDV-299-798-230). All participants signed an informed consent form witnessed by the local health office before collection of data and blood samples HIV [16,17].The questionnaire used to collect demographic and epidemiological data was anonymous and linked to the blood sample tube only by a code, as previously described [11].
Prevalence of Hepatitis D Virus: a total of 1911 patients with a national number (i.e. Libyan citizens) were initially included in the study but 38 (1.98%) were excluded due to lack of personal information. HBV testing was done uniformly. Of 1873 HBsAg-positive samples studied, 34 samples were positive for HDV, yielding an overall HDV prevalence of 1.7%. Of the 34 positive cases, 15 (44.1%) were females and 19 (55.9%) were males. No significant relationship between sex and HDV positivity was evident (P = 0.001). The mean age was 47.1 ± 13.4 years (median = 45.0). The highest HDV prevalence was among persons aged over 60 years (n = 13; 38.2%) followed by those aged 40-60 years (n = 11; 32.4%) and 21-40 years (n =7; 20.6%). It was lowest among those below 20 years of age (n = 3; 8.8%). The distribution of HDV seropositivity differed markedly from one region to another. The prevalence was higher in the central region (P < 0.001). Overall, HDV seropositivity was nearly twice more likely in the central region than in the other regions, and particularly compared to the eastern region.
Study population characteristics: Table 1 shows the demographic and risk factor features in the study population. A variety of risk factors were found to be significantly associated with HDV infection. HDV seropositivity was more prevalent among those with no formal education (41.2%, P < 0.001) than among those with primary or secondary level education. Furthermore, marital status, family history of HBV infection and sex were found to be less associated with HDV seroprevalence (P = 0.001). A higher prevalence was found among those who had a history of intravenous drug use (35.3%; P < 0.001), HCV infection (17.6%) or HIV infection (23.5%, P <0.001. On the other hand, only two patients had a history of blood transfusion.
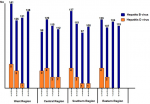
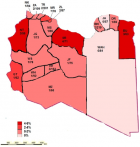
Epidemiological patterns and geographic distribution: Figure 1 shows the annual frequencies of HBV and HDV diagnoses. The overall prevalence was higher in the central region (P < 0.001). In the western region, the highest rate was reported in 2015, after which it declined and reached zero in 2018. In the central region, the highest rates were in 2015 and 2016, after which the rate became steady in 2017 and 2018. In the southern region, the highest rate of HDV seropositivity was reported in 2015, after which it declined steadilyy until 2018. In the eastern region, HDV was reported only in 2015 and 2016. Figure 2 shows the geographic distribution of HDV in all Libyan districts. Seropositivity rates varied markedly from one district to another. Particularly high rates of sero-prevalence were reported in three districts. These include Sert (SR; 5.6%) in the central region, Nalout (NT; 5.1%) in the western region, and Butnan (BT; 4.8%) in the eastern region. These were followed by the districts of Margeb (MR) and Zletan (ZT) in the central region and Ghat (GT) in the southern region. The lowest seroprevalence rates were found in Murzak (MZ), Sebha (SB), Wadi Haiat (WH) and Wadi Shati (WS) in the southern region, followed by Jfara (JR), Musrata (MR) in the central region, and Tripoli, Zawia, Nuqat al Khams (NK) 1/94=1.1 and Ajfra in the western region. No HDV-positive cases were detected in Jabel Alkder (JK) and Wahat (WAH) in the eastern region.
In this large comprehensive nationwide study we analyzed the sero-prevalence of HDV among 1873 HBsAg-positive individuals collected from all the regions and districts of the country during four years. Anti-Delta antibodies was observed in all age groups, with an overall prevalence of 1.7%. The highest rate was reported among those aged over 40 years and the lowest among those aged below 20 years. This 1.7% prevalence rate is very low compared with other Arab countries, such as Saudi Arabia (13.6% ), Mauritania (9-67%) and the neighboring countries Egypt (23.53%), Tunisia, (7-44% ) and Sudan (25-28%) [1,18,19].The prevalence of HDV infection in our cohort study was similar to the findings from other Mediterranean countries, including Spain and France, in which very low rates (<5%) were reported among chronic HBsAg-positive individuals [20,21]. This low rate of HDV infection in Libya, particularly among young adults, was expected because HBV vaccination has been strongly encouraged and offered free of charge in Libya since 1989 and it became compulsory for infants (3 months of age) and children (≥ 12 years of age). Furthermore, viral screening is mandatory in Libya for pregnant women, before surgical operations, and before a marriage contract is concluded. These measures have contributed immensely to reducing viral transmission within the Libyan population [11]. Notably, the use of highly effective anti-HBV vaccines has decreased both the prevalence and the spread of HBV and its associated pathogen, HDV. This has led to the belief that HDV eradication is approaching.
By analyzing the demographic characteristics contributing to the emergence of HDV in Libya, evidence of familial transmission, influence of sex and prior hospitalization were not found to be significant. Moreover, no particular risk factor was found to be associated with HDV co-infection. Injection drug use was the most probable route of HDV transmission for most of the patients in our study (P < 0.001). Those coinfected with HIV or HCV accounted for 23.5% and 17.6%, respectively. In contrast, in Europe, prevalence rates of HDV antibodies approaching 15% have been reported among HBsAg-positive carriers with HIV coinfection. 21 In Taiwan, a highly endemic region, incident HDV superinfections continue to occur. The prevalence rate of anti-HDV seropositive intravenous drug users among anti-HIV seronegative and anti-HIV seropositive cases was 40.0% and 84.2%, respectively [22]. The distribution of HDV diverged in the regions of the country and among distinct within the same region. HDV was reported in all Libyan districts, except for Jabel Alkder (JK) and Wahat (WAH). The central region had the highest prevalence of HDV. Of the 34 cases of detected in this study, 11 (32.4%) came from the central region, mainly from the districts of Sert, Murgab and Zleitan. In the central region, the highest rate was reported in 2016 and 2015, but it was steady in 2017 and 2018. The second region of a high HDV seropositivity was the western region, from where 26.5% of the cases came, and particularly from Nalut. The third region accounting for HDV prevalence was the southern region (23.5%), where there was a uniform distribution of HD cases among the districts, apart from Ghat. The eastern region had the lowest rate of HDV (17.6%), with most of the cases located mainly in Butnan, neighboring Egypt.
The observed geographic variation in the prevalence of HDV in this study could be related to “micro-epidemiology” in different areas of the country. Large regional and district variation in the prevalence of HDV has previously been reported in other African countries, such as Ethiopia. Aberra et al. (2018) has shown that certain geographical areas in Ethiopia, such as Amhara, Addis Ababa and Afar, appeared to have a higher HDV prevalence than the rates reported in Oromia, SNNPR and Tigray, though the overall prevalence in Ethiopia was reported to be low [23]. In our study, there was a significantly higher prevalence of HDV infection in certain Libyan districts, particularly in the central and western regions. However, the numbers were small and these observations need confirmation in a population-based survey and further investigation are needed to reveal whether specific genetic or cultural factors influence HDV transmission [24-26]. Libya has experienced a major population shifts in 2011. Over 4% of the population were displaced due to internal conflict and a major exodus of African immigrants who had been residing towards the European Union countries [27-29]. Studies on viral hepatitis among African immigrants in Libya have shown that the prevalence of HBV ranged from 8 to 25%, depending on the country of origin. Hence, surveillance of HDV, universal access to HBV vaccination, and improvements in socioeconomic and educational status for both local citizens and immigrants remain the keystone for HDV control strategies [30-32].
This work represents the first large-scale countrywide analysis of the sero-prevalence of HDV in Libya, which is one of the largest countries in North Africa. However, the unselected nature of our study population, in which all patients were screened for HBV and HDV infection independently of clinical manifestations or laboratory abnormalities, might have played a role in generating false positive results [33,34]. Our study shows a current very low rate of HDV infection among chronic HBsAg-positive individuals in Libya. This prevalence is diverse among regions and districts, displaying the highest rates in the central region. However, this study did not evaluate the HBV load within the studied population, which might have underestimated the true HDV prevalence. Further studies are needed to analyze the genetic diversity of HDV, and a screening policy for HDV should be implemented, at least among patients with hepatic liver diseases, immigrants, and groups at a higher risk [35-39].
What is known about this topic
- This study is a national surveillance study carried on HDV infection in Libya, the second largest Country in Africa;
- The prevalence of HDV varies geographically and over time within the Libyan regions and districts;
- Different demographic factors were found to contribute to the prevalence of HDV in Libya.
What this study adds
- Libya in low endemic country regarding HDV infection;
- HIV and IVDUs are the main contributing factors in HDV in Libya;
- Hepatitis D virus screening should be implanted in Libya.
The authors declare no competing interests.
Conceived and designed the experiments: Mohamed Ali Daw performed the experiments: Mohamed Ali Daw and Nadia Emhemed Mohamed Sifennasr analyzed the data: Mohamed Ali Daw, Amina Mohamed Daw contributed reagents/materials/analysis tools: Mohamed Ali Daw and Mohamed Ahmed, Wrote the paper: Mohamed Ali Daw designed the analysis: Mohamed Ali Daw performed cartography: Mohamed Ali Daw and Abdallah El-Bouzedi. Provided advice and critically reviewed the manuscript: Mohamed Ali Daw, Abdallah El-Bouzedi and Mohamed Ahmed. All authors have read and approved the final manuscript.
We are deeply grateful to the Libyan Study Group of Hepatitis& HIV, the Department of Medical Microbiology, Faculty of Medicine, and the Department of Infectious Diseases, Tripoli Medical Centre for their help and support.
Table 1: demographic characteristics and risk factors among HDV-infected patients in Libya 2015-2018
Figure 1: the annual number of cases of hepatitis D and hepatitis B infections in Libya 2015-2018
Figure 2: geographic distribution of hepatitis D virus infections in Libya 2015-2018
- Daw MA, Daw AM, Sifennasr NE, Draha A M, Daw AM et al. The Epidemiology of Hepatitis D Virus in North Africa: a systematic review and meta-analysis. Scientific World Journal. 2018 Sep 26;2018:9312650. PubMed | Google Scholar
- Koh C, Heller T, Glenn JS. Pathogenesis of and new therapies for hepatitis D. Gastroenterol. 2019 Jan 1;156(2):461-76. PubMed | Google Scholar
- Thomas E, Yoneda M, Schiff ER. Viral hepatitis: past and future of HBV and HDV. Cold Spring Harb Perspect Med. 2015;5(2):a021345. PubMed | Google Scholar
- Rizzetto M, Ciancio A. Epidemiology of hepatitis D. In Semin Liver Dis. 2012; 32:211-219. PubMed | Google Scholar
- Noureddin M, Gish R. Hepatitis delta: epidemiology, diagnosis and management 36 years after discovery. Curr Gastroenterol Rep. 2014; 16(1):365. PubMed | Google Scholar
- Farci P, Niro GA, 2018. Current and Future Management of Chronic Hepatitis D. Gastroenterol & Hepatol. 2018; 14(6):p342-351. PubMed | Google Scholar
- Katja Giersch, Maura Dandri. Hepatitis B and delta virus: advances on studies about interactions between the two viruses and the infected hepatocyte. J clinic translational hepatol. 2015; 3(3): 220-229. PubMed | Google Scholar
- Niro GA, Smedile A, Fontana R, Olivero A, Ciancio A, Valvano MR et al. HBsAg kinetics in chronic hepatitis D during interferon therapy: on-treatment prediction of response. Aliment Pharmacol Ther. 2016;44(6):620-628. PubMed | Google Scholar
- Stockdale AJ, Chaponda M, Beloukas A, Phillips RO, Matthews PC, Papadimitropoulos A et al. Prevalence of hepatitis D virus infection in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Global health. 2017; 5(10):e992-e1003. PubMed | Google Scholar
- Komas NP, Ghosh S, Abdou-Chekaraou M, Pradat, P, Al Hawajri N, Manirakiza A et al. Hepatitis B and hepatitis D virus infections in the Central African Republic, twenty-five years after a fulminant hepatitis outbreak, indicate continuing spread in asymptomatic young adults. PLoS Negl Trop Dis. 2018; 12(4):e0006377. PubMed | Google Scholar
- Daw MA, El-Bouzedi A. Prevalence of hepatitis B and hepatitis C infection in Libya: results from a national population-based survey. BMC Infect Dis. 2014; 14:17. PubMed | Google Scholar
- Daw MA, Sifennasr NE, El-Bouzedi A, Daeke AO, Dau AA, Drah et al. Epidemiology of hepatitis B virus in immigrants crossing to Europe from North and Sub-Saharan Africa. Travel Med Infect Dis.2017; 16: 59-61. PubMed | Google Scholar
- Daw MA, El-Bouzedi A, Dau AA. Libyan armed conflict 2011: mortality, injury and population displacement. Afr J Emerg Med. 2015; Sep 1;5(3):101-7. Google Scholar
- Daw MA, El-Bouzedi A, Dau AA. The assessment of efficiency and coordination within the Libyan health care system during the armed conflict-2011. Clin Epidemiol Glob Health. 2016;4(3):120-127. Google Scholar
- Sandrine François-Souquière, Maria Makuwa , Ulrich Bisvigou, Mirdad Kazanji . Epidemiological and molecular features of hepatitis B and hepatitis delta virus transmission in a remote rural community in central Africa. Infect Genet Evol. 2016;39:12-21. PubMed | Google Scholar
- Daw MA, Ali LAB, Daw AM, Sifennasr NE, Dau AA, Agnan et al. The geographic variation and spatiotemporal distribution of hepatitis C virus infection in Libya: 2007-20 BMC Infect Dis. 2018; 18(1):594. PubMed | Google Scholar
- World Medical Association. WMA Declaration of Helsinki: ethical principles for medical research involving human subjects.. Google Scholar
- Njoh J, Zimmo S. Prevalence of antibody to hepatitis D virus among HBsAg-positive drug-dependent patients in Jeddah, Saudi Arabia. East Afr Med J. 1998; 75(6):327-328. PubMed | Google Scholar
- Spearman CW, Afihene M, Ally R, Apica B, Awuku Y, Cunha L et al. Hepatitis B in sub-Saharan Africa: strategies to achieve the 2030 elimination targets. Lancet Gastroenterol Hepatol. 2017; 2(12):900-909. PubMed | Google Scholar
- Ordieres C, Navascués CA, González-Diéguez ML, Rodríguez M, Cadahía V, Varela M et al. Prevalence and epidemiology of hepatitis D among patients with chronic hepatitis B virus infection: a report from Northern Spain. Eur J Gastroenterol Hepatol. 2017; 29(3):277-283. PubMed | Google Scholar
- Sellier PO, Maylin S, Brichler S, Berçot B, Lopes A, Chopin D et al. Hepatitis B Virus?Hepatitis D Virus mother-to-child co-transmission: a retrospective study in a developed country. Liver Int. 2018; 38(4):611-618. PubMed | Google Scholar
- Hsieh MH, Wang SC, Hsieh MY, Huang CF, Yeh ML, Yang JF et al. Hepatitis D virus infections among injecting drug users with and without human immunodeficiency virus infection in Taiwan. Kaohsiung J Med Sci. 2016; 32(10):526-530. PubMed | Google Scholar
- Aberra H, Gordien E, Desalegn H, Berhe N, Medhin G, Mekasha B et al. Hepatitis delta virus infection in a large cohort of chronic hepatitis B patients in Ethiopia. Liver Int. 2018; 38(6):1000-1009. PubMed | Google Scholar
- Pinho-Nascimento CA, Bratschi MW, Höfer R, Soares CC, Warryn L, Pečerska J et al. Transmission of Hepatitis B and D Viruses in an African Rural Community. mSystems. 2018; 3(5):e00120-18. PubMed | Google Scholar
- Le Gal F, Brichler S, Drugan Alloui T, Roulot D, Pawlotsky JM, Dény P et al. Genetic diversity and worldwide distribution of the deltavirus genus: a study of 2,152 clinical strains. Hepatol.2017; 66(6).1826-1841. PubMed | Google Scholar
- Daw MA, El-Bouzedi AA, Ahmed MO, Dau AA, Agnan MM, Drah AM. Geographic integration of hepatitis C virus: a global threat. World J Virol. 2016; 5(4):170-172. PubMed | Google Scholar
- Daw MA, El-Bouzedi A, Ahmed MO, Dau AA, Agnan MM. In association with the Libyan Study Group of Hepatitis; HIV: epidemiology of hepatitis C virus and genotype distribution in immigrants crossing to Europe from North and sub-Saharan Africa. Travel Med Infect Dis. 2016; 14: 517-526. PubMed | Google Scholar
- Daw MA, El-Bouzedi A, Ahmed MO, Dau AA, Agnan MM, Drah AM et al. Prevalence of human immune deficiency virus in immigrants crossing to Europe from North and Sub-Saharan Africa. Travel Med Infect Dis.2016;14:637-8. PubMed | Google Scholar
- Daw MA, El-Bouzedi A, Dau AA. Geographic distribution of HCV genotypes in Libya and analysis of risk factors involved in their transmission. BMC Res Notes. 2015;8:367. PubMed | Google Scholar
- Daw MA. The Epidemiology of Hepatitis C virus infection in African immigrants; bridging the gaps. Travel Med Infect Dis. 2018; 27:130. PubMed | Google Scholar
- Daw MA, El-Bouzedi A, Ahmed MO, Dau AA, Agnan MM. Hepatitis C virus in North Africa: an emerging threat. Sci World J. 2016;2016:7370524. PubMed | Google Scholar
- Daw MA, El-Bouzedi AH, Dau AA. Trends and patterns of deaths, injuries and intentional disabilities within the Libyan armed conflict: 2012-2017. PloS one. 2019 May 10;14(5):e0216061. PubMed | Google Scholar
- Wang Y, Glenn JS, Winters MA, Shen LP, Choong I, Shi YL et al. A new dual-targeting real time RT-PCR assay for Hepatitis D Virus RNA Detection. Diagn Microbiol Infect Dis. 2018;92(2):112-117. PubMed | Google Scholar
- Ricco G, Popa DC, Cavallone D, Iacob S, Salvati A, Tabacelia D et al. Quantification of serum markers of hepatitis B (HBV) and Delta virus (HDV) infections in patients with chronic HDV infection. J Viral Hepat. 2018 Aug;25(8):911-919. PubMed | Google Scholar
- Safaie P, Razeghi S, Rouster SD, Privitera I, Sherman KE. Hepatitis D diagnostics: utilization and testing in the United States. Virus Res. 2018 May 2; 250:114-117. PubMed | Google Scholar
- Koh C, Heller T, Glenn JS. Pathogenesis of and New Therapies for Hepatitis D. Gastroenterol. 2019 Jan;156(2):461-476e1. PubMed | Google Scholar
- Jackson C, Gunson RN, Bradley?Stewart A, Bennet S, Black H, Kennedy N et al. Epidemiology and patient characteristics of hepatitis D virus infection in the West of Scotland 2011-2016. J Viral Hepat. 2018 Nov;25(11):1395-1396. PubMed | Google Scholar
- Aguilera A, Trastoy R, Rodríguez-Calviño J, Manso T, de Mendoza C and Soriano V. Prevalence and incidence of hepatitis delta in patients with chronic hepatitis B in Spain. Eur J Gastroenterol Hepatol. 2018 Sep;30(9):1060-1062. PubMed | Google Scholar
- Daw MA, Daw AM, Sifennasr NE, Draha AM, Daw AA, Daw AA et al. Spatiotemporal analysis and epidemiological characterization of the human immunodeficiency virus (HIV) in Libya within a twenty-five-year period: 1993-2017. AIDS Res Ther. 2019 Jun 25;16(1):14. PubMed | Google Scholar