Performance of malaria rapid diagnostic test in febrile under-five children at Oni Memorial Children’s Hospital in Ibadan, Nigeria, 2016
Nurudeen Ayobami Adebisi, Hannah Odunola Dada-Adegbola, Magbagbeola David Dairo, IkeOluwapo Oyeneye Ajayi, Olufemi Olamide Ajumobi
Corresponding author: Nurudeen Ayobami Adebisi, Nigeria Field Epidemiology and Laboratory Training Program, Abuja, Nigeria 
Received: 05 Jul 2017 - Accepted: 17 Feb 2018 - Published: 01 Aug 2018
Domain: Public health
Keywords: Malaria, rapid diagnostic test, microscopy, parasitemia, sensitivity, specificity
©Nurudeen Ayobami Adebisi et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Nurudeen Ayobami Adebisi et al. Performance of malaria rapid diagnostic test in febrile under-five children at Oni Memorial Children’s Hospital in Ibadan, Nigeria, 2016. Pan African Medical Journal. 2018;30:242. [doi: 10.11604/pamj.2018.30.242.13268]
Available online at: https://www.panafrican-med-journal.com//content/article/30/242/full
Original article 
Performance of malaria rapid diagnostic test in febrile under-five children at Oni Memorial Children’s Hospital in Ibadan, Nigeria, 2016
Performance of malaria rapid diagnostic test in febrile under-five children at Oni Memorial Children’s Hospital in Ibadan, Nigeria, 2016
Nurudeen Ayobami Adebisi1,&, Hannah Odunola Dada-Adegbola2, Magbagbeola David Dairo3, IkeOluwapo Oyeneye Ajayi1,3, Olufemi Olamide Ajumobi1,4,5
1Nigeria Field Epidemiology and Laboratory Training Program, Abuja, Nigeria, 2Department of Medical Microbiology and Parasitology, College of Medicine, University of Ibadan, Nigeria, 3Department of Epidemiology and Medical Statistics, College of Medicine, University of Ibadan, Nigeria, 4African Field Epidemiology Network, Abuja, Nigeria, 5National Malaria Elimination Programme, Federal Ministry of Health, Abuja, Nigeria
&Corresponding author
Nurudeen Ayobami Adebisi, Nigeria Field Epidemiology and Laboratory Training
Program, Abuja, Nigeria
Introduction: the World Health Organization (WHO) recommends testing of suspected malaria cases before treatment. Malaria rapid diagnostic test (mRDT) has been recommended for this purpose in endemic countries where microscopy is not accessible. However, its diagnostic performance remains a concern in clinical settings. We assessed diagnostic performance of RDT among febrile under-five children (U5) presenting at Oni Memorial Children's Hospital, Ibadan (OMCH).
Methods: a cross-sectional study was conducted among 370 febrile U5 attending OMCH February to May, 2016. We examined their finger prick blood samples for malaria parasitaemia using CareStartTM histidine rich protein II (HRP-2) RDT and microscopy. The sensitivity, specificity, positive and negative predictive values (PPV, NPV), false positive (FP), invalid rates (IR), likelihood ratio of positive and negative tests (LRP and LRN), were calculated.
Results: mean age of the children was 28.17 ± 15.59 months. Malaria prevalence was 21.6% and 15.1% by mRDT and microscopy, respectively. Sensitivity of CareStartTM HRP-2 RDT was 94.6% (95% confidence interval (CI): 84.2-98.6), specificity: 91.4% (CI: 87.6-94.2), PPV: 66.3% (CI: 54.7-76.2), NPV: 98.9% (CI: 96.8-99.7), FPR 6.5%, IR 8.1%, LRP:10.6 and LRN:0.1.
Conclusion: diagnostic performance of CareStartTM used in the study met the ≥ 95% sensitivity at 100 parasites/µL recommended by WHO. This finding provides clinical evidence that testing before anti-malarial treatment as recommended by WHO will identify cases of malaria infection and reduce unnecessary use of drugs. Healthcare workers should be educated on diagnostic accuracy of mRDT and adhere to the WHO's test-treat strategy for anti-malaria therapy.
Malaria is a life threatening tropical disease responsible for significant morbidity and mortality especially, among children and pregnant women, with 48 to 304 million new cases of malaria infections reported annually worldwide. Nigeria accounted for up to 29% of the total estimated malaria cases and 26% of deaths in the African region [1]. Accurate diagnosis of malaria still remains a challenge because of lack of pathognomonic symptoms, as fever which is one of the commonest symptoms of the disease in children has numerous causes. Infection with bacteria, viruses, protozoa or fungi can manifest as febrile illnesses, thereby presenting with common overlapping manifestations making clinical diagnosis very difficult and leading to undue delay in initiating treatment of fever in children, with the attendant complications including deaths [2]. In the tropics and malaria-endemic regions, most fevers are presumed to be due to malaria and are treated empirically as such [3]. There is mounting evidence that a significant proportion of these febrile illnesses are non malaria [4, 5]. These non-malarial febrile illnesses have been defined as infectious diseases in patients who present with undifferentiated fever and require malaria rapid diagnostic tests or microscopy, but in whom these tests were negative [6]. Despite the awareness of non malaria causes of febrile illness, empirical treatment of fevers, with antimalarial medicines continues in resource-poor settings. In order to limit the development of resistance to drugs and mitigate the consequences of failure of therapy, the World Health Organization advocate for test-based management of malaria and restricting artemisinin-based combination therapy (ACT) to only parasitologically confirmed cases [7]. Diagnosis of malaria based on blood film microscopy using thick and thin smears stained with Giemsa has remained the gold standard for many years [8, 9]. However, the inherent limitations of microscopy such as non-availability of technical expertise and erratic electricity supply and dependence on sophisticated equipment have severely hindered its universal routine use particularly in a busy outpatient clinic and rural settings [10].
This necessitated the introduction of mRDT kits in the early 1990s with the potential to overcome the weaknesses associated with microscopy [11]. Malaria rapid diagnostic tests are based on malaria antigen detection and are meant to differentiate malaria from non-malarial febrile illness, with a turnaround time of less than thirty minutes and have been shown to be effective [12]. CareStartTM malaria an HRP2-based immunochromatographic test which detects HRP2 antigen that is specific for Plasmodium falciparum has a very high performance level as reported by the most recent mRDT evaluation programme [13] and is among the mRDTs approved for procurement by National Malaria Elimination Programme (NMEP). Nigeria adopted mRDT as a diagnostic tool, where microscopic diagnosis is not feasible [14], however, the diagnostic performance of the mRDT and its routine usage has remained a source of concern to health-care providers in clinical settings [15, 16]. Some of the challenges include erratic supply, non-availability of test kits and health care worker's perspectives about its accuracy and reliability with resultant non-adherence to test result when used [15, 16]. This may not be totally unconnected with varying reports of sensitivity of different types of mRDT kits ranging from 94.3% in Ibadan [17], 47.0% in Port Harcourt [18], 40.3% in Zamfara [19] to as low as 8.3% in Maiduguri [20]. The level of sensitivity of the different mRDT kits reported in majority of the studies fall far below the recommended level of ≥ 95% sensitivity by WHO. Therefore, the poor use of CareStartTM RDT despite availability at a secondary level hospital, Oni Memorial Children's Hospital Ibadan (OMCH) could largely be as a result of varying reports of sensitivity in Nigeria. We therefore assessed the diagnostic performance of CareStartTM RDT among febrile under-five children (U5) who presented at OMCH.
Study area: a hospital-based cross-sectional study carried out at Oni Memorial Children's Hospital, Ibadan in Ibadan South West Local Government area, Nigeria from February to May, 2016. Oni Memorial Children's Hospital was purposively selected because it is the only secondary level health facility dedicated to children below 10 years in Oyo State. The hospital is a 65-bedded capacity facility, consists of an emergency unit, intensive care, out-patient unit and 2 specialised units for Heamatology Day Care and Neurological cases. It serves as a referral center for all the health centers within and outside Ibadan. It also provides routine immunization and child welfare services. An average of 900 children of which 500 (55.6%) are under-five are attended to on a monthly basis, while about 750 (83%) of these children are presumptively diagnosed as malaria cases thereby necessitating laboratory confirmation. Though, CareStartTM RDT was available at the OMCH, microscopy remained the mainstay of diagnosing malaria.
Sample size estimation: the number of participants for the study was calculated using the following formula:
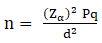
[21] where: n = minimum number of participants needed; Zα = standard normal deviate corresponding to 2-sided level of significance at 5% = 1.96; p = prevalence of malaria parasitaemia among under-five children presenting with malarial at a general hospital in Ota, Ogun State, 70% [22]. q = 1 - p; d = level of precision at 5%
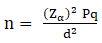
(1.96)2 x 0.70 x 0.30/(0.05)2 = 323 Adjusting for non-response rate (10%) calculated using the following formula:
= n x 1/(1-r) Where: n = sample size calculated; r = non response rate (10%); = 323 x 1/(1-0.1) = 359. The minimum sample size for the study was 370.
Recruitment of participants and data collection: a total of 370 febrile under-five children who presented at the emergency and general out-patient (GOP) units of the OMCH with history of fever (axillary temperature > 37.5°C) and a provisional diagnosis of malaria and whose parent/caregivers gave informed consent for the study were recruited consecutively over a period of two months. We excluded all children who were severely ill and because we could not analyze the drug level of those who have taken any anti-malaria drug, they were also excluded. Data on demographic characteristics, child characteristics and episodes of malaria illness were collected from the caregivers using pre-tested interviewer administered questionnaire.
Laboratory examination: capillary blood sample of each febrile child obtained by finger prick was collected on frosted end slides and CareStartTM Histidine-rich protein 2 (HRP-2) RDT Cassette. Thick and thin blood films were prepared, stained with Giemsa same day and examined microscopically according to WHO standards [23]. Parasite density per microliter of blood (parasitaemia) was determined according to the number of parasites per 200 white blood cells (WBC), assuming a total WBC count of 8,000 /μL and expressed as follows: Parasite density µL-1 = parasite count/number of white blood cells counted × 8000 Microscopic examination of all stained slides was conducted by a trained WHO certified malaria microscopist with 7 years of experience, following standard methods [23]. Each sample was tested with CareStartTM malaria Pf RDT, Lot No. M015C01 manufactured in March, 2015 by Access Bio Incorporation, New Jersey, USA. This was obtained directly from the pharmacy Unit of OMCH through United State agency for International Development (USAID) representative. All the participants were tested by adhering strictly to manufacturer's guidelines.
Quality control: the positive control check of the RDT was done with positive (dilutions at 200 and 2000 parasite/µL of blood) and negative (zero parasitaemia) confirmed by microscopy.
Data analysis: data was analyzed using Statistical Package for Social Sciences (SPSS) software version 16.0. Data were summarized using mean, frequencies, proportions and 95% confidence interval (CI). Parameters for mRDT diagnostic performance was calculated using the standard WHO format as follows: (a) True positive (TP) = sample is positive by both microscopy and CareStartTM HRP-2; (b) True negative (TN) = sample is negative by both microscopy and CareStartTM HRP-2; (c) False positive (FP) = sample is positive by CareStartTM HRP-2 but negative by microscopy; (d) False negative (FN) = sample is negative by CareStartTM HRP-2 but positive by microscopy.
Ethical consideration: ethical approval for the study was obtained from Oyo State Ethical review committee (reference number: AD13/479/144, date: 13/01/2016). Informed consent was also obtained from the caregivers.
Overall, 370 febrile children were included in the analysis. The mean age of the children was 28.2 months (standard deviation: 15.6). The male participants constitute 211 (57.0%), other children characteristics are shown in Table 1. The most common presenting complaint other than fever was loss of appetite (23.2%), cough and catarrh (22.4%) as well as body weakness (15.4%). While all the participants presented with history of fever, only 123 (33.2%) was febrile (temperature ≥ 37.5°C) at time of presentation (Table 1). The prevalence of malaria or the proportion of positive cases among the febrile U5 were 21.6% (80/370) and 15% (56/370) by mRDT and microscopy, respectively (Table 2). The CareStartTM HRP-2 detected 53 true positives (TP), 27 false positives (FP), 287 true negatives (TN) and 3 false negatives (FN). The microscopy detected 56 malaria positive cases among the participants, 55 Plasmodium falciparum and 1 Plasmodium malariae, while the RDT detect 80 positive cases of Plasmodium falciparum (Table 2). The Sensitivity of CareStartTM HRP-2 RDT was 94.6% (95% CI: 84.2-98.6) and specificity of 91.4% (CI: 87.6-94.2) (Table 3).
Despite several interventions, malaria still continues to be a major public health challenge especially among under five children. Malaria parasitaemia prevalence of 15.1% and 21.6% was observed with light microscopy and mRDT respectively in this study. This observed prevalence is lower than previously reported prevalence among children presenting at health facilities in Nigeria which ranged between 27.7% and 84.7% in studies by Ikeh and Teclaire [24], (56.9%), Oladehinde et al [22, 25], (70.0%,84.7%), Okoli and Solomon [26] (51.0%) and Elechi et al [20] (27.7%). The differences in the prevalence rates may be due to some reasons which include, but not limited to, the seasonal variation, location of the study (rural or urban). However, wide spread use of ACTs in the Country, insecticide treated nets (ITN) and other preventive measures may have contributed to the low malaria prevalence reported in our study, which is in line with a declining trend in malaria prevalence in Nigeria [27, 28]. Therefore, febrile U5 children should not be assumed to have malaria without testing. While, testing before antimalarial will reduce the abuse or over prescription of antimalarial drugs and will allow early diagnosis of other cause of febrile illness. CareStartTM RDT HRP2 Pf shows a good performance with high sensitivity of 94.6% and comparable to the 94.3% and 93.0% sensitivity reported in Ibadan by Falade et al [17] and in Sokoto by Sani et al [29] respectively. However, it varied markedly from sensitivity of 100% ,78.4%, 69.6%, 42.31% and 40.3% reported in Kaduna by Ajumobi et al [30], Jos by Sheyin and Bigwan [31], Lagos by Ben-Edet et al., [32], Enugu by Oguonu and Okafor [33], and Zamfara by Abdulkadri et al [19], respectively.
The disparity in reported sensitivity may not be because the studies used different kits, for instance while Ajumobi, Falade, and Jeremiah all used SD Bioline malaria Pf RDT, they still reported discordant sensitivity of 100%, 94.3% and 47% respectively. Also for the CareStartTM RDT HRP2 Pf, while the current study gave a sensitivity of 94.6%, Sheyin and Bigwan, and Abdulkadri reported 78.4% and 40.3% respectively. These observed conflicting reports may be due to the functionality of RDTs which remains unable to detect parasites at low densities (< 200-400/ µL). However, other factors such as storage which were not considered in the current study may be responsible for the differences which has eroded the confidence of end users. Although the RDT specificity was high (91.4%), 27 children still had false positive results while microscopy was negative. This may imply that the children did not have active malaria, or had incomplete treatment with antimalarial drug, however, the positive RDT result could be attributed to delayed clearance of antigen from the circulation. It has been shown that malaria antigen clearance could be delayed for up to 4 weeks even, when treatment was successful Abdulkadri et al [19]. High specificity of RDT kits will reduce unnecessary treatment with antimalarial drug and improve early diagnosis of other causes of febrile illnesses in all settings. Furthermore, the three observed children with false negative result may suggest that the children were infected with Plasmodium species other than falciparum or caused by other non-HRP2 containing P. falciparum. The current finding of 98.9% negative predictive value of CareStartTM also support the validity on its use in excluding/eliminating malaria as the cause of febrile illness and allows early decision making in looking out for other causes of febrile illness in the under-fives, which will enhance their survival, thereby reducing mortality in under-fives. The other findings which include invalid rate of 8.1% are within the acceptable rates according to WHO assessment of RDTs 2014-2015 round six [13].
The diagnostic performance of CareStartTM HRP-2 RDT met the ≥ 95% sensitivity at 100 parasites/µL recommended by WHO. The study provides the clinical evidence to justify its use for testing before treatment as recommended by WHO. Use of RDT should be encouraged for diagnosis of malaria using a protocol that encourage further evaluation of febrile children with negative RDT results using microscopy. Efforts should be made to educate healthcare workers on the importance of RDT test kits and enforcement of protocols that supports the WHO's test-treat strategy for anti-malaria therapy. There should be regular communication of availability of mRDTs among all stakeholders in every health care facilities and also Government at both federal, state and local levels should limit the number of mRDT product types for the country based on local data on performance. Also the varying sensitivities in different parts of Nigeria calls for more study to unravel the circumstances affecting the performance of the rapid test kits.
Limitations of the study: report on the use of antimalarial drug before presentation cannot be verified but we elicited this from the caregivers as much as possible. The negative results could not be confirmed by polymerase chain reaction (PCR) because of limited funds.
What is known about this topic
- Accurate diagnosis of malaria remains a challenge in Nigeria and empirical treatment of fevers, with antimalarial medicines continues in resource-poor settings;
- Diagnosis of malaria based on blood film microscopy using thick and thin smears stained with Giemsa has remained the gold standard for many years;
- Nigeria adopted mRDT as a diagnostic tool, where microscopic diagnosis is not feasible but there have been varying reports of sensitivity of different types of mRDT kits ranging from 8.0 % to 94.3% in Nigeria.
What this study adds
- Diagnostic performance of CareStartTM HRP-2 RDT met the ≥ 95% sensitivity at 100 parasites/µL recommended by WHO;
- The study provides the clinical evidence to justify its use for testing before treatment as recommended by WHO;
- Government at both federal, state and local levels should conduct lot testing of batches of mRDT on a regular basis to ensure the mRDT in use at health facilities are of high diagnostic performance.
The authors declare no competing interest.
All the authors contribute to concept and design, data analysis and statistical interpretation, development of manuscript except data collection. All authors have read and agree to the final manuscript.
We acknowledge and appreciate the staff and patients of OMCH and the Assistant Director Public Health, Oyo state, Dr T.O. Ladipo. The malaria rapid test kit used in this study was provided at no cost by USAID. This study was supported by Cooperative Agreement Number (GH15-1619) U2GGH001876, funded by the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
Table 1: clinical presentation, fever duration and care seeking for the children under five with history of fever at OMCH, Oyo State, February to May, 2016 (N = 370)
Table 2: distribution of cases of malaria detected by parasitological technique in children with history of fever at OMCH, Oyo State, February to May, 2016
Table 3: diagnostic performance of CareStart TM HRP-2 RDT among children with history of fever
- World Health Organization (WHO). Factsheet on the world malaria report. 2016.
- Hofer M, Mahlaoui N, Prieur AM. A child with a systemic febrile illness-differential diagnosis and management. Best Pract Res Clin Rheumatol. 2006; 20(4): 627-640. PubMed | Google Scholar
- D'Acremont V, Lengeler C, Mshinda H, Mtasiwa D, Tanner M, Genton B. Time to move from presumptive malaria treatment to laboratory-confirmed diagnosis and treatment in African children with fever. PLoS Med. 2009; 6(1): e252. PubMed | Google Scholar
- Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, Eisele T, Liu L, Mathers C. Global, regional and national causes of child mortality in 2008: a systematic analysis. The Lancet. 2010; 375(9730): 1969-1987. PubMed | Google Scholar
- D'Acremont V, Lengeler C, Genton B. Reduction in the proportion of fevers associated with Plasmodium falciparum parasitaemia in Africa: a systematic review. Malar J. 2010; 9: 240. PubMed | Google Scholar
- Acestor N, Cooksey R, Newton PN, Menard D, Guerin PJ, Nakagawa J, Christophel E, Gonzalez IJ, Bell D. Mapping the aetiology of non-malarial febrile illness in southeast Asia through a systematic review-terra incognita impairing treatment policies. PLoS One. 2012; 7(9): e44269. PubMed | Google Scholar
- World Health Organization, Guidelines for the Treatment of Malaria, Geneva, Switzerland, 3rd edition, 2015. Google Scholar
- McGregor IA, Wilson RJ. Specific immunity acquired in man; In: Wernsdorfer WH, McGregor IA, editors; Malaria. the Principles and Practice of Malariology; Edinburgh: Churchill Livingstone. 1988; 1: 735-751.
- Murray CK, Gasser RA, Magill AJ, Miller RS. Update on rapid diagnostic testing for malaria. Clin Microbiol Rev. 2008; 21(1): 97-110. PubMed | Google Scholar
- Iwelunor J, Airhihenbuwa C, King G, Adedokun A. Contextualizing child malaria diagnosis and treatment practices at an outpatient clinic in Southwest Nigeria: a qualitative study. ISRN Infect Dis. 2013; 2013: 1-6. Google Scholar
- Murray CK, Bennett JW. Rapid diagnosis of malaria. Interdiscip Perspect Infect Dis. 2009; 2009: 415953. PubMed | Google Scholar
- Hopkins H, Bebell l, Kambale W, Dokomajilar C, Rosenthal PJ, Dorsey G. Rapid diagnostic tests for Malaria at sites of varying transmission intensity in Uganda. J Infect Dis. 2008; 197(4): 510-518. PubMed | Google Scholar
- WHO. Malaria Rapid Diagnostic Test Performance-Result of WHO product testing of malaria RDTs round 6; 2014-2015.
- Federal Ministry of Health (FMOH, 2005) National malaria control programme: revised 5-years strategic plan: 2006-2010. Abuja, Nigeria: Federal Ministry of Health; 2005. Federal Ministry of Health (Nigeria).
- Awoleye OJ, Thron C. Improving access to malaria rapid diagnostic test in Niger State Nigeria: an assessment of implementation up to 2013. Malar Res Treat. 2016; 2016: 7436265. PubMed | Google Scholar
- Boadu NY, Amuasi J, Ansong D, Einsiedel E, Menon D, Yanow S. Challenges with implementing malaria rapid diagnostic tests at primary care facilities in a Ghanaian district: a qualitative study. Malar J. 2016; 15: 126. PubMed | Google Scholar
- Falade CO, Ajayi IO, Nsungwa-Sabiiti J, Siribié M, Diarra A, Sermé L, Afonne C, Yusuf OB, Gansane Z, Jegede AS, Singlovic J, Gomes M. Malaria Rapid Diagnostic Tests and Malaria Microscopy for Guiding Malaria Treatment of Uncomplicated Fevers in Nigeria and Prereferral Cases in 3 African Countries. Clin Infect Dis. 2016; 63(Suppl 5): S290-297. PubMed | Google Scholar
- Jeremiah ZA, Uko EK, Buseri FI, Jeremiah TA. Field Evaluation of SD Bioline Rapid Malaria Diagnostic Test among Asymptomatic Malaria Infected Children in Port Harcourt, Nigeria. Res J Parasitol. 2007; 2(1): 39-44. Google Scholar
- Abdulkadir I, Rufai H, Ochapa S, Malam M, Garba B, Oloko G, George I. Malaria rapid diagnostic test in Children: the Zamfara, Nigeria experience. Niger Med J. 2015; 56(4): 278-282. PubMed | Google Scholar
- Elechi HA, Rabasa AI, Muhammad FB, Garba MA, Abubakar GF, Umoru MA. Prevalence and pattern of malaria parasitaemia among under-five febrile children attending peadiatric out-patient clinic at University of Maiduguri teaching hospital, Maiduguri. Niger J Paediatr. 2015; 42(4): 319-324. Google Scholar
- Lwanga SK, Lemeshow S. Sample size determination in health studies, a practical manual. World Health Organization. 1991; 1-22. Google Scholar
- Olasehinde GI, Ojurongbe DO, Akinjogunla OJ, Egwari LO, Adeyeba AO. Prevalence of Malaria and Predisposing Factors to Antimalarial Drug Resistance in Southwestern Nigeria. Res J Parasitol. 2015; 10(3): 92-101.
- WHO Basic malaria microscopy part 1: Learner's guide. WHO, Geneva, 1991.
- Ikeh EI, Teclaire NN. Prevalence of malaria parasitaemia and associated factors in febrile under-5 children seen in primary health care centers in Jos, North Central Nigeria. Niger Postgrad Med J. 2008; 15(2): 65-69. PubMed | Google Scholar
- Olasehinde GI, Ajayi AA, Taiwo SO, Adekeye BT, Adeyeba OA. Prevalence and management of falciparum malaria among infants and children in Ota, Ogun State, Southwestern Nigeria. Afr J Clin Exper Microbiol. 2010; 11(3): 159-163. Google Scholar
- Okoli C, Solomon M. Prevalence of Hospital-Based Malaria among Children in Jos, North Central Nigeria. Br J Med Med Res. 2014; 4(17): 3231-3237. Google Scholar
- National Population Commission (NPC) (Nigeria), National Malaria Control Programme (NMCP) (Nigeria) and ICF International. Nigeria Malaria Indicator Survey 2010. Abuja, Nigeria: NPC, NMCP and ICF International. 2012.
- National Malaria Elimination Programme (NMEP), National Population Commission (NPopC), National Bureau of Statistics (NBS) and ICF International. Nigeria Malaria Indicator Survey 2015. Abuja, Nigeria and Rockville, Maryland, USA: NMEP, NPopC and ICF International. 2016.
- Sani U, Jiya N, Ahmed H. Evaluation of a malaria rapid diagnostic test among febrile children in Sokoto, Nigeria. Int J Med Sci. 2013; 3: 433-440.
- Ajumobi O, Kabir S, Patrick N, Jacob K, Godwin N, Sheba G, Rutebemberwa E, Wellington O, Peter N, Mark M, Gabriele P. Performance of an HRP- 2 rapid diagnostic test in Nigeria children less than 5 years of age. Ame J Trop Med Hyg. 2015; 92(4): 828-833. PubMed | Google Scholar
- Sheyin Z, Bigwan I. Comparison of CARE START HRP2 rapid malaria test with light microscopy for guiding patient's treatment of fever in Nigeria endemic areas. J Med Med Sci. 2013; 4(9): 353-356. Google Scholar
- Ben-Edet AE, Lesi FE, Mafe AG, Grange AO. Diagnosis of falciparum malaria in children using the immuno-chromatographic technique. Niger J Paediatr. 2004; 3(13): 71-78. Google Scholar
- Oguonu T, Okafor HU. Comparison of clinical, microscopic and rapid diagnostic test methods in the diagnosis of Plasmodium falciparum malaria in Enugu, Nigeria. Niger Postgrad Med J. 2007; 14(4): 285-289. PubMed | Google Scholar











