Multiple myeloma associated with an Evanís syndrome
Achour Bechir, Regaieg Haifa, Ben Sayed Nesrine, Bouslema Emna, Mejdoub Senda, Achour Asma, Bouatay Bouzouita Amina, Senda Mrabet, Ben Youssef Yosra, Kortas Mondher, Khelif Abderrahim
Corresponding author: Achour Bechir, Department of Hematology, Farhat Hached Hospital, Sousse Tunisia 
Received: 15 Sep 2016 - Accepted: 17 Oct 2016 - Published: 01 Nov 2016
Domain: Clinical medicine
Keywords: Multiple myeloma, evan´s syndrome, autoimmun hemolytic anemia, thrombocytopenia
©Achour Bechir et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Achour Bechir et al. Multiple myeloma associated with an Evanís syndrome. Pan African Medical Journal. 2016;25:127. [doi: 10.11604/pamj.2016.25.127.10750]
Available online at: https://www.panafrican-med-journal.com//content/article/25/127/full
Multiple myeloma associated with an Evanís syndrome
Achour Bechir1,&, Regaieg Haifa1, Ben Sayed Nesrine1, Bouslema Emna1, Mejdoub Senda2, Achour Asma2, Bouatay Bouzouita Amina3, Senda Mrabet4, Ben Youssef Yosra1, Kortas Mondher3, Khelif Abderrahim1
1Department of Hematology, Farhat Hached Hospital, Sousse Tunisia, 2Department of Radiology, Farhat Hached Hospital, Sousse Tunisia, 3Laboratory of biologic Hematology, Farhat Hached Hospital, Sousse Tunisia, 4Departement of Nephrology, Sahloul Hospital, Sousse Tunisia
&Corresponding author
Achour Bechir, Department of Hematology, Farhat Hached Hospital, Sousse Tunisia
Auto-immun events are rare in multiple myeloma (MM). Here, we report one MM case complicated by Evans syndrome (Autoimmun hemolytic anemia (AIHA) associated with thrombocytopenia). A 52-year-old man was admitted in nephrology department with severe anemia, renal insufficiency and hypergamma globulinemia. Laboratory exams showed acute hemolysis due to an IgG warm autoantibody. Serum electrophoresis revealed the presence of a monoclonal IgG protein and urinary M protein was 2g/day. A whole body CT-Scan showed osteolytic lesions of vertebral body of C5, D4, L3, L4 and the left iliac wing. The diagnosis of multiple myeloma and Evan's syndrome was made, we underwent chemotherapy by BTD (bortezomib-thalidomide-dexamethasone) and continuous corticosteroid therapy but unfortunately the patient died secondary of a Lactic acidosis. The relationship between MM and hemolysis remain unclear.
Multiple myeloma (MM) is a B-cell malignancy characterized by monoclonal proliferation of plasma cells in the bone marrow. Anemia associated with MM has been reported to arise from disorder of maturation of erythroid lineage, shortening life span of erythrocytes and iron deficiency associated with increased tumor cell mass [1,2], while auto immune hemolytic anemia (AHAI) has been rarely reported. The association between AIHA and MM remained unclear [3]. Here, we report a second case of MM complicated with Evan's syndrome.
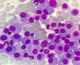
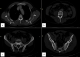
A 52 year's old men with no medical history was admitted in the department of nephrology for 2 months of fatigue, dizziness and acute renal failure: creatinine 330 Ķmol/l and clearance of creat 14 ml /min. No abnormalities were observed in physical examination except conjunctival jaundice, liver and spleen were not palpable. Laboratory investigation showed: hemoglobin 86g/L, red blood cell (RBC) 3.67*1012/L, reticulocytes count of 5.2%, white blood cells (WBC) 12.3*109/L, platelet count 36*109/L, lactate dehydrogenase (LDH) was 451UI/L, indirect bilirubin 49 mmol/L and total bilirubin 96 mmol/L. No hypercalemia was noted. Direct Coomb's test (DCT) was positive for auto-antibody. The autoantibody eluted was warm IgG antibody. At the same time, total serum protein 113 g/L and a spike in gamma globulin estimated to 69 g/l. Serum immune electrophoresis showed that the M protein was the IgG kappa chain. Urinary M protein was 2g/day. Bone marrow aspirates showed infiltration by 78% of plasma cells (Figure 1). Cytogenetics by conventional karyotyping showed no chromosomal abnormalities. A whole body CT-Scan showed osteolytic lesions of vertebral body of C5, D4, L3, L4 and the left iliac wing (Figure 2). After a diagnosis of multiple myeloma and Evan's syndrome was made, chemotherapy was rapidly initiated by BTD (bortezomib-thalidomide-dexamethasone) and continuous corticosteroid therapy. Evolution was marked by deterioration of the hematological and renal state along with the occurrence of hemorrhagic symptoms such as echymosis and haematemesis. The patient died secondary of a lactic acidosis.
The most common complications of multiple myeloma include anemia, hypercalcemia, pathologic fractures, renal failure and recurrent infections. More than two thirds of all patients with MM have anemia [4, 5]. Anemia occurring in patients with MM is multifactorial. Several factors have been implicated in the pathogenesis : Bone marrow (BM) infiltration by malignant plasma cells, hemodilution linked to the hyperprotidemia, Chemotherapy-induced bone marrow suppression, iron deficiency and vitamins, relative erythropoietin (EPO) deficiency (due partly to renal impairment) [6].
†
A rare cause of anemia is hemolysis due to auto immune, drug induced and traumatic-microangiopathic process or flowing intensive chemotherapy [7]. In fact auto-immunity is a current manifestation in other hematological malignancies such as chronic lymphocytic leukemia or non Hodgkin lymphomas and it is extremely rare in MM. Previous studies showed that only about 4% of AIHA patients had myeloma [8]. While others reports have stated that myeloma is rarely associated with hemolytic anemia [8]. Thrombocytopenia is seen frequently in patients with multiple myeloma when most often the etiology is either marrow replacement by the myeloma cells or treatment with anti-myeloma agents. Immune thrombocytopenia is often seen in patients with lymphoproliferative disorders such as non-Hodgkinīs lymphoma and chronic lymphocytic leukaemia. However this is has only rarely been documented in patients with multiple myeloma [9]. In addition, ITP can be associated with AIHA as Evansī syndrome, with hemolysis usually preceding thrombocytopenia [10]. Eight cases of AIHA and one case of Evan's syndrome have been described in patients with MM, in none of these cases the pathogenesis of hemolysis was lucid [5, 11].†
In our patient the diagnosis of MM was clear, both M-protein produced by MM and the immonoglobin that bound to the patient red cell's was IgG type which suggest that the M-protein may has been responsible of the hemolysis, however this could not be confirmed and it needs further investigations. It's unclear wether auto-immune manifestations are results of MM or it's just a coincidence to be present in the same time. Only one study lead by Wada and al showed that M-protein and the Ig directed against red cells was from the same subtype and subtype IgG1kappa [8].†
Also it has been suggested that MM is a B-cell malignancy associated with a marked immune disturbance that may allow normally suppressed clones to develop and produce auto antibodies against red cell surface antigens [12]. Counter selection of VH4-36 segment in MM due to self-tolerance mechanism. Several drugs had also been shown to be causative agent for immune mediated hemolysis in MM such as lenalidomide and interfern α due to immunomodelatery effects [13, 14]. The fast awful outcome of the patient assume that was probably a refractory form of MM associated to Evan's syndrome, as well as Hofer S et al reported the effectiveness of Rituximab in refractory AIHA and MM [15]. So we should have tried the anti CD20 monoclonal antibodies to resolve the situation.
Although auto-immune events occurs rarely in patients with MM, they must be kept in mind. Further studies are necessary to understand the mechanism of hemolysis in this disease.
Authors declare that they have no Competing interests.
All authors contributed to the conception of this case report. All authors also claim to have read and approved the final manuscript. We are grateful to Pr Bouatay Bouzouita Amina, Mejdoub Senda for their contribution in pathology analysis.
Figure 1: multiple myelomaĖ cytology: magnification x 100: diffuse infiltration of the bone marrow aspiration by mature plasma cells. Most cells display mature chromatin, low nuclear cytoplasmic ratio and flamed cytoplasm
Figure 2: cervical-thoracic-lumbo-sacral and pelvis CT scan: (A, B, C, D) diffuse lytic lesions and sclerotic bony lesions in iliac bones
- Silvestris F, Cafforio P, Tucci M, Dammacco F. Negative regulation of erythroblast maturation by Fas-L(+)/TRAIL(+) highly malignant plasma cells: a major pathogenetic mechanism of anemia in multiple myeloma. Blood. 2002; 99(4):1305-13. PubMed | Google Scholar
- Patel R, Salamassi S. Possible auto-immune hemolysis with multiple myeloma: a case report with review of the literature. Blood. 1987; 70( suppl 1):113a. PubMed | Google Scholar
- Vaiopoulos G, Kyriakou D, Papadaki H, Fessas P, Eliopoulos GD. Multiple myeloma associated with autoimmune hemolytic anemia. Haematologica. 1994; 79(3): 262-4. PubMed | Google Scholar
- Sergentanis TN, Zagouri F, Tsilimidos G, Tsagianni A, Tseliou M, Dimopoulos MA, Psaltopoulou T. Risk Factors for Multiple Myeloma: a Systematic review of meta-analyses. Clin Lymphoma Myeloma Leuk. 2015; 15(10): 563-77. PubMed | Google Scholar
- Shah BK, Uprety D, Tretheway D. Multiple myeloma presenting with autoimmune hemolytic anemia. Indian J Hematol Blood Transfus. 2014; 30(Suppl 1): 38-9. PubMed | Google Scholar
- Benjilali L, Essaadouni L. A multiple myeloma associated with autoimmune hemolytic anemia. Presse Med. 2013; 42(11):1533-5. PubMed | Google Scholar
- Mittelman M. The implications of anemia in multiple myeloma. Clin Lymphoma. 2003; 1 (suppl 4): S23-9. PubMed | Google Scholar
- Wada H, Yata K, Mikami M, Suemori S, Nakanishi H, Kondo T, Tsujioka T, Suetsugu Y, Otsuki T, Sadahira Y, Yawata Y, Sugihara T. Multiple myeloma complicated by autoimmune hemolytic anemia. Intern Med. 2004; 43(7): 595-8. PubMed | Google Scholar
- Gupta V, Hegde UM, Parameswaran R, Newland AC. Multiple myeloma and immune thrombocytopenia. Clin Lab Haematol. 2000; 22(4): 239-42. PubMed | Google Scholar
- Cines DB, Liebman H, Stasi R. Pathobiology of secondary immune thrombocytopenia. Semin Hematol. 2009; 46(1 Suppl 2): S2-14. PubMed | Google Scholar
- Yi Y, Zhang GS, Gong FJ, Yang JJ. Multiple myeloma complicated by Evans syndrome. Intern Med J. 2009; 39(6): 421-2. PubMed | Google Scholar
- Kashyap R, Singh A, Kumar P. Prevalence of autoimmune hemolytic anemia in multiple myeloma: a prospective study. Asia Pac J Clin Oncol. 2016; 12(2): e319-22. PubMed | Google Scholar
- Darabi K, Kantamnei S, Wiernik PH. Lenalidomide induced warm autoimmune hemolytic anemia. J Clin Oncol. 2006; 24(35): e59. PubMed | Google Scholar
- Gesundheit B, Zelig O, Shapira MY, Ackerstein A, Avgil M, Or R. Complete remission of multiple myeloma after autoimmune hemolytic anemia: possible association with interferon-alpha. Am J Hematol. 2007; 82(6): 489-92. PubMed | Google Scholar
- Hofer S, Hunziker S, Dirnhofer S, Ludwig C. Rituximab effective in a patient with refractory autoimmune haemolytic anaemia and CD20-negative multiple myeloma. Br J Haematol. 2003; 122(4): 690-1. PubMed | Google Scholar