Infective endocarditis presenting as acute coronary syndrome
Tahar El Kandoussi, Hicham El Malki, Alae El Masmoudi, Meryam Loubaris, Mohamed Laaroussi, Mohamed Cherti
Corresponding author: Tahar El Kandoussi, Department of Cardiology B, Maternity Hospital, Rabat, Morocco 
Received: 05 Jul 2015 - Accepted: 10 Apr 2016 - Published: 26 Apr 2016
Domain: Clinical medicine
Keywords: Acute coronary syndrome, vegetation embolization, paravalvular abscess
©Tahar El Kandoussi et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Tahar El Kandoussi et al. Infective endocarditis presenting as acute coronary syndrome. Pan African Medical Journal. 2016;23:230. [doi: 10.11604/pamj.2016.23.230.7429]
Available online at: https://www.panafrican-med-journal.com//content/article/23/230/full
Infective endocarditis presenting as acute coronary syndrome
Tahar El Kandoussi1,&, Hicham El Malki2, Alae El Masmoudi1, Meryam Loubaris2, Mohamed Laaroussi2, Mohamed Cherti1
1Department of Cardiology B, Maternity Hospital, Rabat, Morocco, 2Department of Cardiovascular Surgery A, Ibn Sina Hospital, Rabat, Morocco
&Corresponding author
Tahar El Kandoussi, Department of Cardiology B, Maternity Hospital, Rabat, Morocco
We report tow cases of infective endocarditis (IE) presenting as acute coronary syndrome (ACS). Case 1: A 60-year-old man with the diagnosis of mitral IE complicated by an ST segment elevation myocardial infarction. Primary percutaneous coronary intervention with aspiration of the thrombus at the distal leftanteriordescending (LAD) arterywas successfully performed. Case 2: A 72 year old man was admittedfor an aortic root abscess compressing the left coronary artery. The treatment required surgery, including coronary artery bypass procedure. The postoperative course was complicated by multiple organ failure, and the patient died after 48 h.
ACSis a rare complication of IE, and is most commonly due to coexisting coronary disease. More rarely, emboli from vegetations may give rise to infarction. Mechanical compression of coronary arteries from perivalvular extension of aortic endocarditis is exceptional. Early recognition of IE as a potential etiology of arterial occlusion is paramount in tailoring further investigations, and instituting appropriate management.
1stcase
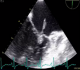
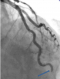
A 60-year-old man, presented with severe chest pain, persistent fever and arthralgia for one month prior to admission. His coronary risk factors were hypertension and current smoking. He had no significant past medical history. Empiric treatment with amoxicillin/clavulanicacidfor2weeks was initiated at another clinic, but his fever had persisted. On admission, his blood pressure was 120/70 mm Hg, pulse rate was 105 beats/min. Physical examination revealed a systolic regurgitant murmur over the mitral valve area. Initial transthoracic echocardiography showed moderate mitral regurgitation, and an oscillating mass 14×3 mm on mitral valve. The diagnosis ofmitral valve IE was confirmed by transoesophageal echocardiography (TEE); there was no annular abscess (Figure 1). The results of laboratory tests are shown as follows: the white blood cell count was 14,500 mm3, hemoglobin concentration 9.3 g/l, creatinine 92 μmol/l and C-reactive protein 123 mg/dl. Computed topographics can of the head revealed no abnormalities. Initial blood cultures grew staphylococcus aureus. Antibiotic treatment with Oxacillin + gentamicin was started. His symptoms improved within 72 hours later. Four days after admission, he felt a sudden onset of substernal chest pain. His ECG showed ST segment elevation in leads V3 V4, and emergency echocardiography revealed the disappearance of vegetation on the mitral valve. The coronary angiography noted a complete occlusion of the distal tract of the LAD artery, while the remaining coronary arteries were completely free from any stenosis/atherosclerotic lesion (Figure 2). After aspiration of the thrombus, the distal LAD occlusion resolved with restoration of TIMI flow grade 3. His complaints and ECG changes improved after the procedure, and he was discharged after the completion of 6 weeks of antibiotic treatment.
2nd case
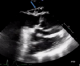
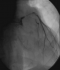
A 72-year-old man, with a history of rheumatic heart disease, presented with a 2-month history of fever and lethargy. His coronary risk factors were age and hypertension. Laboratory tests revealed significant inflammatory syndrome (CRP=178 mg/l) and anemia (hemoglobin=10.1 g/dl). Renal function was correct. Sensitive Staphylococcus aureus was isolated fromperipheral blood cultures. Transthoracic echocardiography showed a large circumferential periaortic abscess, and a doppler revealed moderate aortic regurgitation. TEE confirmed the same data(Figure 3). The patientwas diagnosed withIE, and treated with intravenous flucloxacillin and gentamicin. Computed tomographic scan of the head showed no abnormalities, and abdominal computed tomography scan found a mycotic aneurysm of a branch of the superior mesenteric artery measuring 13 mm (Figure 4). Three days after admission, the patient complained of worsening chest pain. The electrocardiogram showed significant anterior ST depression, and troponin I level rose to 5.3 ng/ml. Emergency coronary angiography noted an area of narrowing of the left main and LAD arteries (Figure 5). The patient was transferred to the cardiac surgery for further management. Emergency surgery confirmed a large aortic root abscess compressing the left main and LAD arteries. The patient underwent an obliteration of the abscess cavity, aortic valve replacementwith a bioprosthesis, annulus reconstruction using pericardium, and a single aortocoronary bypass graft using the left internal mammary artery. There was considerable difficulty in weaning the patient of by-pass, and despite intra-aortic balloon pumping, high dose catecholamine support and antibiotics, the patient died after 48h.
IE is still associated with high in-hospital mortality, ranging from 16% to 25%, and a high incidence of embolic events. Systemic embolism is a common complication of IE, most frequently involving the central nervous system, spleen, kidney and liver. Whereas ACS is infrequently encountered. The incidence of coronary septic embolism is difficult to estimate. Only 2.9% of 586 Spanish patients had ACS, 1.5% of cases occurred with native valves, and embolism was the cause in only 0.51% of patients [1]. In a recent study, increased troponin T levels in patients with IE were a predictor of increased mortality and cerebrovascular accident [2]. In our first case, such as the most cases described in the literature, coronary embolisms occur in theLAD artery, because of the downward course of this artery compared with the right coronary artery or left circumflex artery [1]. Therefore, the infarction is anterior or anterolateral. Septic emboli are more frequent with mitral valve infection (25%) than with aortic valve infection (10%) [3]. ACS in patients with IE is more often associated with virulent microorganisms. In both cases, we noted that Staphylococcus areus is the causative agent of IE. While Staphylococcus species have increased risk of abscess formation and embolization, a recent review of the literature found that streptococcus species were the most common organisms isolated in cases of embolism causing infarction [4]. Clinical presentation of ACS in patients with IE are similar to those observed in individuals with coronary atherosclerosis. Coronary angiography can establish the diagnosis of septic emboli in the coronary artery. However, contact between the catheter and the valve surface with vegetation may release systemic emboli [5]. The optimal management strategy for reperfusion in these patients remains unclear. Thrombolytics and complimentary antithrombotic regimens may greatly increase the risk of intracerebral hemorrhage [5].
Balloon or stent procedures mayallow mycotic aneurysm to develop at the site, resulting in complications including coronary rupture or sudden death [6]. It was reported that the stenting is probably associated with higher risk of mycotic aneurysm formation than balloon angioplasty alone, because the vegetation is jailed between the vessel wall and the stent [7]. Surgery would be indicated if a coronary mycotic aneurysm were detected, because of its tendency to rupture. A paravalvular abscess usually presents as persistent fever, despite appropriate antibiotic therapy, or as a new conduction abnormality. Few cases of coronary artery compression associated with an aortic root abscess have been reported. In 1985, Rojo et al reported about the first case of mycotic of the left ventricular outflow tract that impinged on the LAD artery, resulting in angina [8]. The aortic root abscess may be responsible for myocardial ischemia, particularly when it is located between 12 o'clock and 3 o´clock on the aortic annulus. TEE remains the optimum imaging technique to define the complications of aortic valve endocarditis, including formation of root abscess and fistulae [9]. Coronary angiography can demonstrate coronary compression, and is still crucial for evaluating the patient with aortic valve endocarditis and evidence of myocardial ischemia. If periannular complications are present, the patient is referred for surgery. Several surgical series have shown significantly reduced mortality rates when aortic homografts are used in preference to mechanical prostheses [10]. Extrinsic coronary compression by periannular complications is associated with high mortality in patients with IE. Ischemia is often secondary to extrinsic compression of the left main coronary artery or the proximal segment of the LAD artery. In select patients, aortocoronary bypass grafting may be necessary at the time of aortic valve surgery. In our second case, advanced age, importance of sepsis, and persistent ischemia were obvious factors for the poor postoperative evolution.
ACS is an uncommon complication in patients with IE. The mechanism responsible for myocardial ischemia varies, but septic emboli and extrinsic coronary compression by periannular complications are common. Early recognition of IE as a potential etiology of ACS is paramount for instituting appropriate management.
The authors declare no competing interest.
All authors have read and agreed to the final version of this manuscript and have equally contributed to its content and to the management of the case.
Figure 1: transoesophageal echocardiogram, tow -chamber view at 90°, showing the vegetation attached to mitral valve
Figure 2: the left anterior descending artery (LAD) had a total distal occlusion (blue arrow) with an abrupt cut -off and TIMI 0 flow
Figure 3: transoesophageal echocardiogram, at 120° showing a large circumferential abscess at the aortic root (blue arrow), with a thickened aortic valve
Figure 4: abdominal CT scan showing a mycotic aneurysm of a branch of the superior mesenteric artery (black arrow)
Figure 5: coronary angiography showing an area of narrowing of the left main and left anterior descending arteries consistent with compression from an external source
- Manzano MC, Vilacosta I, San Román JA et al. Acute coronary syndrome in infective endocarditis. Rev Esp Cardiol. 2007 Jan; 60(1):24-31. PubMed | Google Scholar
- Rittoo D. Elevation of cardiac troponin T in infective endocarditis predicts an adverse outcome. J Am CollCardiol .2006; 47 SupplA: 280. PubMed | Google Scholar
- Bayer AS, Bolger AF, Taubert KA et al. Diagnosis and management of infective endocarditis and its complications. Circulation. 1998 Dec 22-29; 98(25):2936-48. PubMed | Google Scholar
- Khan F, Khakoo R, Failinger C. Managing embolic myocardial infarction in infective endocarditis: current options. J Infect. 2005 Oct;51(3):e101-5. PubMed | Google Scholar
- Welton DE, Young JB, Raizner AE et al. Value and safety of cardiac catheterization during active infective endocarditis. Am J Cardiol. 1979 Dec; 44(7):1306-10. PubMed | Google Scholar
- McGee MB, Khan MY. Ruptured mycoticaneurysm of a coronary artery: a fatal complication of Salmonella infection. Arch Intern Med. 1980 Aug;140(8):1097-8. PubMed | Google Scholar
- ErtanUral, Ulaş Bildirici, GökselKahraman, Baki Komsuoğlu. Coronary embolism complicating aortic valve endocarditis: treatment with successful coronary angioplasty. Int J Cardiol. 2007 Jul 31;119(3):377-9. PubMed | Google Scholar
- Rojo H, Cabrera Fischer E, Weinschelbaum E, Crottengini A, de la Fuente L, Favaloro R. A rare case of coronary artery obstruction. J Thorac Cardiovasc Surg. 1985Mar; 89(3):448-50. PubMed | Google Scholar
- Vilacosta I, Camino A, Sarria et al. Mechanical compression of the left coronary artery resulting from periannular extension of aortic endocarditis: diagnosis by transesophageal echocardiography. Am Heart J. 1994 Oct;128(4):823-7. PubMed | Google Scholar
- Tuna IC, Orszulak TA, Schaff HV et al. Results of homograft aortic valve replacement for active endocarditis. Ann ThoracSurg. 1990 Apr;49(4):619-24. PubMed | Google Scholar