Endogenous hyperinsulinism: diagnostic and therapeutic difficulties
Esma Leila Gouta, Hichem Jerraya, Wejih Dougaz, Mohamed Ali Chaouech, Ibtissem Bouasker, Ramzi Nouira, Chadly Dziri
Corresponding author: Esma Leila Gouta, Surgical Department B, Charles Nicolle Hospital, Faculty of Medicine of Tunis, University of Tunis El Manar, Tunis, Tunisia 
Received: 20 Apr 2019 - Accepted: 17 May 2019 - Published: 27 May 2019
Domain: Endocrinology,Internal medicine,General surgery
Keywords: Insulinoma, hypoglycemia, endogenous hyperinsulinism
©Esma Leila Gouta et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Esma Leila Gouta et al. Endogenous hyperinsulinism: diagnostic and therapeutic difficulties. Pan African Medical Journal. 2019;33:57. [doi: 10.11604/pamj.2019.33.57.18885]
Available online at: https://www.panafrican-med-journal.com//content/article/33/57/full
Endogenous hyperinsulinism: diagnostic and therapeutic difficulties
Esma Leila Gouta1,&, Hichem Jerraya1, Wejih Dougaz1, Mohamed Ali Chaouech1, Ibtissem Bouasker1, Ramzi Nouira1, Chadly Dziri1
1Surgical Department B, Charles Nicolle Hospital, Faculty of Medicine of Tunis, University of Tunis El Manar, Tunis, Tunisia
&Corresponding author
Esma Leila Gouta, Surgical Department B, Charles Nicolle Hospital, Faculty of Medicine of Tunis, University of Tunis El Manar, Tunis, Tunisia
Endogenous hyperinsulinism is an abnormal clinical condition that involves excessive insulin secretion, related in 55% of cases to insulinoma. Other causes are possible such as islet cell hyperplasia, nesidioblastosis or antibodies to insulin or to the insulin receptor. Differentiation between these diseases may be difficult despite the use of several morphological examinations. We report six patients operated on for endogenous hyperinsulinism from 1st January 2000 to 31st December 2015. Endogenous hyperinsulinism was caused by insulinoma in three cases, endocrine cells hyperplasia in two cases and no pathological lesions were found in the last case. All patients typically presented with adrenergic and neuroglycopenic symptoms with a low blood glucose level concomitant with high insulin and C-peptide levels. Computed tomography showed insulinoma in one case out of two. MRI was carried out four times and succeeded to locate the lesion in the two cases of insulinoma. Endoscopic ultrasound showed one insulinoma and provided false positive findings three times out of four. Intra operative ultrasound succeeded to localize the insulinoma in two cases but was false positive in two cases. Procedures were one duodenopancreatectomy, two left splenopancreatectomy and two enucleations. For the sixth case, no lesion was radiologically objectified. Hence, a left blind pancreatectomy was practised but the pathological examination showed normal pancreatic tissue. Our work showed that even if morphological examinations are suggestive of insulinoma, other causes of endogenous hyperinsulinism must be considered and therefore invasive explorations should be carried out.
Endogenous hyperinsulinism is an abnormal clinical condition that involves excessive insulin secretion. It is related in 55% of cases to insulinoma which should be first evoked in each case of endogenous hyperinsulinism [1]. Other causes are possible such as islet cell hyperplasia, nesidioblastosis or antibodies to insulin or to insulin receptor [2]. Differentiation between these different etiologies may be difficult especially in cases where morphological examinations are negative [3].
We report here six patients operated on for endogenous hyperinsulinism while underlining diagnostic and therapeutic difficulties.
It is a retrospective and descriptive study that included six consecutive patients admitted at the Surgical Department B of Charles Nicolle Hospital for endogenous hyperinsulinism, from 1st January 2000 to 31st December 2015, and operated on with the most likely diagnosis of insulinoma.
Data were presented by their median and range values.
Six cases of endogenous hyperinsulinism were enrolled over a period of 15 years. All clinical features were summarized in Table 1.
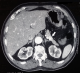
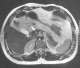
Were included four females and two men with a median age of 48.5 years, ranging from 31 to 82 years. Endogenous hyperinsulinism was caused by insulinoma in three cases, endocrine cells hyperplasia in two cases and no pathological lesions were found in the last case. All patients typically presented with adrenergic and neuroglycopenic symptoms. The median time between the first occurrence of symptoms and the diagnosis of endogenous hyperinsulinism was 12 months (range 3-36). All patients had a low blood glucose level concomitant with a high insulin level. The C-peptide level was measured only in five patients. It was high in four cases and normal in one case. Computed tomography (CT) showed insulinoma in one case out of two (Figure 1). Magnetic resonance imaging (MRI) was carried out four times and succeeded to locate the lesion in the two cases of insulinoma (Figure 2). Endoscopic ultrasound (EUS) showed one insulinoma and provided false positive findings in three times out of four. Intra operative ultrasound (IOUS) succeeded to localize the insulinoma in two cases but was false positive in two cases.
Regarding the localization of the detected lesions, procedures were: one duodenopancreatectomy, two left splenopancreatectomy and two enucleations. For the sixth case, no lesions were objectified neither by preoperative imaging nor by IOUS. Hence, a left blind pancreatectomy was practised. However, histological examination was normal.
Symptoms of hypoglycemia disappeared for all patients postoperatively. Pancreatic fistula was observed in two cases which dried spontaneously. In five cases out of six, no clinical signs of recurrence were observed after a median follow up of 12 months (range 3-24). In case n°2 (Table 1) corresponding to islet cell hyperplasia, symptoms of endogenous hyperinsulinism recurred one year after and were biologically confirmed. However, imaging explorations (ultrasound (US), CT, MRI and EUS) were negative. A distal pancreatic resection was performed. The postoperative course was uneventful with normalization of blood glucose level. Nevertheless, pathological examination revealed normal pancreatic tissue. She was lost to follow up after 24 months.
Our work showed that, firstly, endogenous hyperinsulinism was associated only in three cases to an insulinoma out of six and secondly, the other causes of endogenous hyperinsulinism must be considered even if morphological examinations are suggestive of insulinoma.
Insulinoma is the most frequent cause of endogenous hyperinsulinism since it is found in 55% of cases in adults [1]. Its incidence is about 1-4 per million [2]. The aim of preoperative imaging is to localize the insulinoma. However, preoperative topographic diagnosis of insulinoma remains a difficult challenge because of low sensitivity concerning conventional imaging techniques for pancreatic small lesions. Indeed 80% of insulinoma have less than 2cm in diameter and 40% less than 1cm [3]. This explains the reported sensitivities of CT and MRI which are respectively about 33%-64% and 40%-90% [4, 5]. In our work, MRI was the most efficient method to detect insulinoma whereas EUS revealed false positive findings three times out of four. EUS can yield misleading findings and its sensitivity to detect insulinoma is about 80% [3]. Mabrut reported in his study a case with a false positive diagnosis of insulinoma related to postoperative fibrosis [3]. Kann also showed that pancreatic nodules of unknown dignity were localized in 1% of 438 cases obtained by EUS [6].
When insulinoma is clinically and biologically evoked, some authors believe that negative imaging explorations do not affect the indication of surgical exploration associated with IOUS [3]. In our study, IOUS succeeded to localize the insulinoma in two cases but was false positive in two cases. De Santibañes revealed that IOUS allowed the detection of the pancreatic tumor from 92% to 94.7% of cases [7]. However, according to Mabrut, the sensitivity of IOUS was 86.3% and the false positive cases were related to postoperative scar fibrosis [3].
Other authors used aggressive strategies to localize the insulinoma in case of negative investigations. Stimulation of insulin secretion in response to calcium injection into a pancreatic artery called Arterial Stimulation Venous Sampling, helps to detect the pancreatic tumor in 90% of cases [8]. It had a sensitivity of 87.5% [9]. Moreover, scintigraphy GLP -1R agonists were recently developed to optimize both pre-operative and intraoperative location of insulinoma with promising results [10]. When insulinoma is not localized, particularly intraoperatively, some authors argue that the procedure should be stopped and the patient referred to a centre capable to perform advanced preoperative and intraoperative localization techniques mentioned above [10, 11]. This would avoid blind distal pancreatectomy for occult insulinoma. In our study, we performed one blind distal pancreatectomy, but the pathological examination was normal. Nikfarjam reported three failed blind distal pancreatic resections for insulinoma. Later on, the three patients were found to have tumors within the pancreatic head [12].
Furthermore, our study suggests that the other causes of endogenous hyperinsulinism should be considered if the results of preoperative morphological imaging are negative or discordant. Endocrine cells hyperplasia is defined by an expansion of the endocrine cell mass to more than 2% in adults [13]. Histologically, all types of islet cells are normally distributed throughout the islets but the predominant cell type is the β cell [13]. Beta cells hyperplasia can only be controlled by partial pancreatectomy, which was done for one of our two patients with a successful result. The second case who had enucleation presented a recurrence of hypoglycemia one year after surgery. The other cause of endogenous hyperinsulinism is nesidioblastosis which represents 0.5 to 7% of all adults [14]. It is revealed clinically by neuroglycopenic and catecholamine response symptoms during fasting. In contrast to patients with insulinoma, such symptoms may occur post prandially [14]. Documented hyperinsulinemic hypoglycemia with a clinically and chemically negative fast test is highly suggestive of nesidioblastosis [15]. Surgical treatment with pancreatectomy is the recommended therapeutic method [2]. Finally, endogenous hyperinsulinism may also be caused by a high titre of antibodies to insulin or by insulin receptor mutations. The autoimmune syndrome is revealed by initial hyperglycemia followed by hypoglycemia a few hours later and biologically with high insulin levels, usually above 100 mU/L [16]. Insulin receptor mutations are revealed by increased fasting levels of serum insulin with normal C-peptide levels. Therapeutic management of both hyperinsulinism caused by autoimmune syndrome or by insulin receptor mutations is not well defined [17].
Endogenous hyperinsulinism is an abnormal clinical condition that involves excessive insulin secretion, related in 55% of cases to insulinoma. Other causes are possible such as islet cell hyperplasia, nesidioblastosis or antibodies to insulin or to the insulin receptor. Differentiation between different etiologies of hyperinsulinism may be difficult especially when morphological examinations are negative. Our work showed that even if morphological examinations are suggestive of insulinoma, other causes of endogenous hyperinsulinism must be considered and therefore invasive explorations should be carried out.
What is known about this topic
What this study adds
The authors declare no competing interests.
Esma Leila Gouta and Jerraya Hichem participated in the conception and design, acquisition of data, analysis and interpretation of data. Bouasker Ibtissem, Wejih Dougaz and Chaouech Mohamed Ali participated in revising it critically for important intellectual content. Chadli Dziri and Ramzi Nouira participated in the design of the study and performed the statistical analysis. All authors read and approved the final manuscript.
Table 1: clinical presentation of the six cases of endogenous hyperinsulinism
Figure 1: abdominal CT scan showed a hypervascular lesion of 20mm localized in the tail of the pancreas
Figure 2: T2-weighted MRI of the abdomen showed a hypervascular lesion of 10mm localized in the head of the pancreas
- Phan GQ, Yeo CJ, Hruban RH, Lillemoe KD, Pitt HA, Cameron JL. Surgical experience with pancreatic and peripancreatic neuroendocrine tumors: review of 125 patients. Journal of Gastrointestinal Surgery. 1998; 2(5):473-482. PubMed | Google Scholar
- Jabri AL, Bayard C. Nesidioblastosis associated with hyperinsulinemic hypoglycemia in adults: review of the literature. European journal of internal medicine. 2004; 15(7):407-410. PubMed | Google Scholar
- Mabrut JY, Lifante JC, Cherki S, Sin S, Berger N, Peix JL. La localisation préopératoire des insulinomes est-elle utile? In: Annales de chirurgie. Elsevier. 2001; 126(9):850-856. PubMed | Google Scholar
- Service FJ, McMahon MM, O'brien PC, Ballard DJ. Functioning insulinoma-incidence, recurrence, and long-term survival of patients: a 60-year study. In: Mayo Clinic Proceedings. Elsevier. 1991 Jul; 66(7):711-719. PubMed | Google Scholar
- McAuley G, Delaney H, Colville J, Lyburn I, Worsley D, Govender P, et al. Multimodality preoperative imaging of pancreatic insulinomas. Clinical radiology. 2005; 60(10):1039-1050. PubMed | Google Scholar
- Kann PH, Wirkus B, Keth A, Goitom K. Pitfalls in endosonographic imaging of suspected insulinomas: pancreatic nodules of unknown dignity. European journal of endocrinology. 2003; 148(5):531-534. PubMed | Google Scholar
- de Santibañes M, Cristiano A, Mazza O, Grossenbacher L, de Santibanes E, Clariá RS, et al. Endogenous Hyperinsulinemia Hypoglucemic Syndrome: Surgical Treatment. Cirugía Española (English Edition). 2014; 92(8):547-552. PubMed | Google Scholar
- Guettier J-M, Kam A, Chang R, Skarulis MC, Cochran C, Alexander HR, et al. Localization of insulinomas to regions of the pancreas by intraarterial calcium stimulation: the NIH experience. The Journal of Clinical Endocrinology & Metabolism. 2009; 94(4):1074-1080. PubMed | Google Scholar
- Christ E, Wild D, Forrer F, Brandle M, Sahli R, Clerici T, et al. Glucagon-like peptide-1 receptor imaging for localization of insulinomas. The Journal of Clinical Endocrinology & Metabolism. 2009; 94(11):4398-4405. PubMed | Google Scholar
- Hirshberg B, Libutti SK, Alexander HR, Bartlett DL, Cochran C, Livi A, et al. Blind distal pancreatectomy for occult insulinoma, an inadvisable procedure1 1No competing interests declared. Journal of the American College of Surgeons. 2002 Jun 1; 194(6):761-4. PubMed | Google Scholar
- Rostambeigi N, Thompson GB. What should be done in an operating room when an insulinoma cannot be found? Clinical Endocrinology. 2009 Apr; 70(4):512-5. PubMed | Google Scholar
- Nikfarjam M, Warshaw AL, Axelrod L, Deshpande V, Thayer SP, Ferrone CR, et al. Improved Contemporary Surgical Management of Insulinomas. Ann Surg. 2008 Jan; 247(1):165-72. PubMed | Google Scholar
- Rindi G, Solcia E. Endocrine Hyperplasia and Dysplasia in the Pathogenesis of Gastrointestinal and Pancreatic Endocrine Tumors. Gastroenterology Clinics of North America. 2007 Dec 1; 36(4):851-65. PubMed | Google Scholar
- Walmsley D, Matheson NA, Ewen S, Himsworth RL, Bevan JS. Nesidioblastosis in an Elderly Patient. Diabetic Medicine. 1995; 12(6):542-5. PubMed | Google Scholar
- Hirshberg B, Livi A, Bartlett DL, Libutti SK, Alexander HR, Doppman JL, et al. Forty-Eight-Hour Fast: The Diagnostic Test for Insulinoma. J Clin Endocrinol Metab. 2000 Sep 1;85(9):3222-6. PubMed | Google Scholar
- Lupsa BC, Chong AY, Cochran EK, Soos MA, Semple RK, Gorden P. Autoimmune Forms of Hypoglycemia. Medicine. 2009 May; 88(3):141. PubMed | Google Scholar
- Højlund K, Hansen T, Lajer M, Henriksen JE, Levin K, Lindholm J, et al. A Novel Syndrome of Autosomal-Dominant Hyperinsulinemic Hypoglycemia Linked to a Mutation in the Human Insulin Receptor Gene. Diabetes. 2004 Jun 1; 53(6):1592-8. PubMed | Google Scholar