The changing epidemiology of esophageal cancer in sub-Saharan Africa – the case of Ghana
Mark Tettey, Frank Edwin, Ernest Aniteye, Lawrence Sereboe, Martin Tamatey, Ernest Ofosu-Appiah, Innocent Adzamli
Corresponding author: Mark Tettey, National Cardiothoracic Centre, Box K B 846, KBTH. Accra, Ghana 
Received: 17 Mar 2012 - Accepted: 22 Jul 2012 - Published: 07 Sep 2012
Domain: Epidemiology
Keywords: Esophageal cancer, squamous cell carcinoma, adenocarcinoma, dysphagia
©Mark Tettey et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Mark Tettey et al. The changing epidemiology of esophageal cancer in sub-Saharan Africa – the case of Ghana. Pan African Medical Journal. 2012;13:6. [doi: 10.11604/pamj.2012.13.6.1652]
Available online at: https://www.panafrican-med-journal.com//content/article/13/6/full
Original article 
The changing epidemiology of esophageal cancer in sub-Saharan Africa – the case of Ghana
The changing epidemiology of esophageal cancer in sub-Saharan Africa – the case of Ghana
Mark Tettey1,&, Frank Edwin1, Ernest Aniteye1, Lawrence Sereboe1, Martin Tamatey1, Ernest Ofosu-Appiah1, Innocent Adzamli1
1National Cardiothoracic Center, Box KB 846 Korle Bu Teaching Hospital, Accra, Ghana
&Corresponding author
Mark Tettey, National Cardiothoracic Centre, Box K B 846, KBTH, Accra, Ghana
The esophagus, a muscular tube measuring 20-25 cm long and 2-3cm wide serves as a conduit for food and drink. Structurally the esophageal wall is composed of five histological layers: superficial mucosa layer, lamina propria, submucosa, muscularis propria and adventitia. The esophagus descends anterior to the vertebral column through the superior and posterior mediastinum. After traversing the diaphragm at the diaphragmatic hiatus the esophagus extends through the gastroesophageal junction to end at the gastric cardia [1]. Significantly, the esophagus is inaccessible to clinical examination. Clinical diagnosis of an esophageal lesion is thus based on symptoms and imaging studies. Carcinoma of the esophagus starts innocuously in the esophageal mucosa as a painless lesion and progresses to an advanced lesion before symptoms become apparent. The most common presentation of esophageal cancer leading to its diagnosis is progressive dysphagia. The esophagus is capable of accommodating the initial obstruction because it lacks a serosal layer which allows the smooth muscle to stretch. As a result the patient may not manifest dysphagia until the lumen is more than 50 – 60% obstructed by the tumor.
Esophageal cancer is a devastating disease that continues to have less than 10% five year survival despite advances in multimodality therapy [2]. It is the 8th most common malignancy worldwide and the sixth most common cause of cancer-related deaths [3]. This is the only cancer that is recording an increasing incidence all over the world in spite of the treatment modalities currently available for the management of this disease. The incidence of esophageal cancer represents one of the widest variations with a 60 - fold difference between high and low-incidence regions [4]. It is endemic in many parts of the world particularly in developing countries. High prevalence areas include Asia, Southern and Eastern Africa, and Northern France [4].
Racial disparities in esophageal cancer incidence, mortality and histology exist [5]. Esophageal cancer was reported as the 4th leading cause of death in African Americans [2]. The incidence of esophageal squamous cell carcinoma is more than fivefold higher among blacks than among whites in the USA [2]. The prevalence of squamous cell carcinoma in Africa is higher than adenocarcinoma and these epidemiologic observations are important because of the potential contributions to the understanding of the etiology and pathogenesis of esophageal cancer [2]. The purpose of this study is to evaluate the current national trend in the presentation and outcome of esophageal cancer using our institutional experience spanning two decades. The risk factors and the management options adopted in the treatment of the disease are also reviewed.
We retrospectively reviewed all patients who were treated at Ghana’s National Cardiothoracic Centre from 1992 to 2010. Data collected from patients’ case notes included age, sex, duration of symptoms, history of alcohol ingestion and cigarette smoking, staging of extent of disease surgical procedures performed and intraoperative findings for those who underwent surgery. Information regarding the histopathological diagnosis for some of these patients was also recorded.
Statistical Analysis
Continuous data are represented as mean with standard deviation. Categorical data are represented in frequency and proportions. Chi square test was performed to compare the type of carcinoma and the regional distribution in the esophagus.
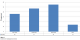
Over the 18 year period, 152 patients with carcinoma of the esophagus were seen. Of these, 122 (80.3%) were males and 30 (19.7%) were females. The age distribution is shown in Table 1. The patients’ age range was 27 to 87 years with mean age of 57.8±11.7 years. The data show an increasing incidence of esophageal carcinoma since 1992. This is shown in Figure 1.
The distribution of esophageal cancer by anatomical location was 5 (3.3%) in the upper third, 18(11.8%) in the middle third and 129 (84.9%) in the distal third. Analyzing 75 patients from the 152 patients in whom histopathological information was available, 78.7% had squamous cell carcinoma and 21.3% had adenocarcinoma. Table 2 shows the histopathological distribution of the lesions. The type of carcinoma and the regional distribution was not significant in this study, p= 0.096.
One hundred and twenty five patients (82%) had history of alcohol ingestion and smoking. Of the 16 cases of adenocarcinoma, 11 (68.8%) had no history of alcohol or smoking; of the 59 cases of squamous cell carcinoma, 6 (10.1%) had no history of alcohol or smoking. These six cases of squamous cell carcinoma occurred exclusively in the middle third of the esophagus. The distribution of squamous cell carcinoma was 45 (76.3%) distal third, 12 (20.3%) middle third and 2 (3.4%) upper third. The most common presentation of esophageal cancer leading to diagnosis was a short history of progressive dysphagia. Sixty-three percent of the patients studied had 1 – 4 months’ history of progressive dysphagia while the duration was 5 – 10 months in the rest. Liver metastasis was present in 40 (36.2%) and lung metastasis in 6 (5.4%) of the patients studied. The type of surgical procedure performed is shown in Table 3. All patients presented with incurable disease. Ninety one (59.9%) of these patients had inoperable tumours; 61 (40.1%) presented with resectable but incurable tumors. Most patients were lost to follow up.
The mean age at diagnosis of esophageal cancer in the current study was 58.4 years with an age range of 27 – 87 years. The bulk of the disease presented in the 50 – 59 year group (Table 1). This is similar to the modal age in a retrospective study carried out in Kenya [6]. The mean age of 3,319 esophageal cancer patients in California was 63.8 years [7]. In Northern Iran where squamous cell carcinoma was not common, the mean age was 61.8±12.0 years [8]. In a systematic review of esophageal cancer in Sub-Saharan Africa, the disease was prominent among the age group of 45 – 65 years in both sexes [9]. In both developed and developing countries the modal age at presentation of esophageal carcinoma appears to be similar. Both squamous cell carcinoma and adenocarcinoma of the esophagus are infrequent before 40 years of age, beyond which the incidence of each type rises with each decade of life. [2] Most of these patients have identifiable risk factors that could be linked with the genesis of the esophageal disease. However, in a recent study in Western Kenya with very high rates of esophageal cancer, an unusual percentage of cases were found in subjects 30 years of age and younger [10]. Majority of these patients had a family history of cancer.
The sex ratio (male to female) all over the world shows significant diversity ranging from 0.85 in Northern Iran [8] to as high as 20.5 in Hispanics [11]. The male to female ratio in our study was 4:1. Other studies have recorded varying figures. [7,9,10,12,13] In a study by Nordenstedt and Seraq, the male to female ratio of esophageal cancer varied according to histology, age and race [11]. In their report, the results showed that for esophageal adenocarcinoma, all races had similar sex- and age- specific incidence patterns with a peak in the male to female ratio in the age group 50 -59 years [11]. The highest male to female ratio was seen in Hispanics (20.5) and the lowest in Blacks (7.0) compared with (10.8) in whites. By contrast the male to female ratios were low and fairly stable throughout the different age groups in esophageal squamous cell carcinoma [11].
The incidence of esophageal cancer has shown a steady increase over the years in this study. The conclusion from the review of esophageal cancer in Sub – Saharan Africa clearly shows that esophageal cancer incidence is on the ascendancy in the sub-region [9]. This is in keeping with our findings and the call for esophageal carcinoma to be included among the diseases of public health importance for effective prevention, early diagnosis and effective treatment is therefore valid.
The most common histopathological type in our study was squamous cell carcinoma (78.7%). This percentage is lower than that in studies from Kenya and South India where over 90% of patients diagnosed with esophageal cancer had squamous cell carcinoma [6,14]. In a study of esophageal cancer epidemiology in blacks and whites, squamous cell carcinoma was more commonly diagnosed in blacks and white females whereas adenocarcinoma was more common among white males [5]. In a study among patients =30 years with esophageal cancer in Kenya, squamous cell carcinoma accounted for 98% of the cases [10]. Less than 20% of these patients took alcohol or smoked cigarette. The peculiarity of squamous cell carcinoma among blacks both in and out of Africa cannot be related to alcohol, smoking and social class alone. As stated by Brown et al in their study, the higher incidence rates observed among blacks for exposure to the same risk factors as whites may reflect a susceptibility state conditioned by genetic traits [15]. There should be some genetic bases to explain this especially when white females have similar predisposition as in blacks.
In Western cultures, retrospective evidence has implicated cigarette smoking and chronic alcohol exposure as the most common etiological factors for squamous cell carcinoma [16]. In this study 82% of the patients took alcohol and smoked cigarette in significant quantities. Tobacco and/or alcohol are known to account for approximately 90% of all esophageal squamous cell carcinoma [17]. Tobacco is linked to esophageal adenocarcinoma but there is no scientific evidence linking alcohol to esophageal adenocarcinoma [17]. Already known is the risk of alcohol and smoking in the causation of esophageal squamous cell carcinoma [3,4,18,19]. Recent studies have shown that alcohol consumption and tobacco use do not affect the risk of esophageal carcinoma in the same way. For alcohol consumption, it is the mean intake (>200g/week) rather than the duration, whereas for tobacco smoking, it is the duration (>15 years) rather than the mean intake that is more closely associated with the risk of esophageal carcinoma [20]. However, the joint effect of alcohol and smoking when consumed together are potentiated and the final relative risk is multiplied [19]. Eighty nine percent of our patients were from low socioeconomic class. Socioeconomic class appears to be an independent risk factor in the development of esophageal carcinoma [2]. The combination of all four risk factors – low social class, moderate/heavy alcohol intake, and infrequent consumption of raw fruits and vegetables – accounted for almost all of the squamous cell carcinoma of the esophagus in one study [15].
The distribution of esophageal cancer by anatomical location was 129 (84.9%) in the distal third, 18 (11.8%) in the middle third and 5 (3.3%) in the upper third. Analyzing 75 of the patients from the 152 patients whose histopathological information were available, 78.7% had squamous cell carcinoma and 21.3% had adenocarcinoma. The distribution of squamous cell carcinoma was 45 (61%) in the distal third, 12 (33%) in the middle third and 2 (6%) in the upper third. Esophageal adenocarcinoma is exclusively a distal third tumor as was the result in this study. The type of carcinoma and the distribution was not significant in this study, p=0.097. This is because in our study, squamous cell carcinoma is more common in the distal third of the esophagus. The diversity of the distribution of esophageal squamous cell carcinoma is demonstrated as some studies have shown that squamous cell carcinoma primarily occurs more in the middle third [2,20]. Other studies are in keeping with our findings where lower esophageal cancers outnumbered the mid esophageal squamous cell carcinoma [8,14]. Interestingly, patients who developed squamous cell carcinoma who neither drank alcohol nor smoked cigarette had their tumor exclusively located in the middle third of the esophagus in this study.
Similar to other studies [16,20-22] we found the most common presentation leading to diagnosis of esophageal cancer was a short history of progressive dysphagia. Sixty three percent of our patients presented with 1 – 4 months history of progressive dysphagia. The absence of serosa and the distensibility of the esophagus delay the symptoms of esophageal cancer until the tumor is advanced. This could contribute to the variable duration of symptoms [6].
The distant nodal disease defined as M1a by TNM staging system could be assessed in 111 patients. Out of these patients 74 (66.7%) had evidence of distant node involvement located around the celiac and/or para-aortic areas. It has been shown that having even one lymph node positive for disease decreases survival rate by 25 - 40% and having more than 4 positive nodes is associated with survival rates of <5% [23]. The distant organ metastasis using chest X-ray, computerized tomography scan, ultrasound scan and intra-operative assessment showed distant metastasis in the liver in 40 (36%) patients and in the lung in 6 (5.4%) patients. PET scan is more accurate in detecting distant metastasis [24,25] though this was not used in this study.
Esophageal cancer generally portends a grim prognosis attributable to late presentation in most patients and all patients in this study presented with incurable disease. Sixty one percent of these patients had inoperable tumors and 39% although resectable were incurable. Patients in this study showed no survival benefit from neoadjuvant therapy. Some underwent initial feeding gastrostomy to prepare for neoadjuvant therapy but most of them deteriorated and could not undergo surgery. Esophageal stenting was done for patients with metastatic disease. In a report by Patel et al, there was no advantage of neoadjuvant therapy over surgery alone [23]. We employed adjuvant chemoradiation after palliative esophagectomy in some selected patients. However, postoperative adjuvant therapy has not been shown to be beneficial [26]. We decided to bypass some selected cases (without metastases, comorbidities, or severe nutritional deficiency) of inoperable tumors with colon and then refer them for adjuvant chemotherapy. Most of these patients did well until their demise from locally advanced or metastatic disease.
The national trend of esophageal carcinoma shows an increasing incidence over the past 2 decades. Alcohol consumption and smoking are major risk factors. Squamous cell carcinoma is the dominant histological type. Late presentation characterized most patients; conventional management did not impact on the survival. Early diagnosis and treatment is advocated but remains an uphill task.
The authors declare no competing interests.
Conceptualization and design of study: Mark Tettey, Frank Edwin, Ernest Aniteye. Data acquisition and analysis: Mark Tettey, Innocent Adzamli, Ernest Ofosu-Appiah, MartinTamatey. Drafting of article: Mark Tettey, Frank Edwin. Critical Revision: Mark Tettey, Frank Edwin, Ernest Aniteye, Lawrence Sereboe. All the authors have read and approved the final version of the manuscript.
Table 1: Age distribution of the 152 patients
Table 2: Histopathological distribution of cancer of the esophagus
Table 3: Type of Surgical Procedure performed
Figure 1: incidence of esophageal carcinoma
- Kuo Braden, Urma Daniela. Esophageal anatomy and development. GI Motility online. 2006; doi:10.1038/gimo6
- Prikens Allan, Orringer Mark. Geographical distribution and racial disparity in esophageal cancer. Ann Thorac Surg. 2003;76:S1367-S1369. This article on PubMed
- Absi Ahmed, Adelstein David, Rice Thomas. Esophageal Cancer. www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/hematology-oncology/esophageal-cancer/..Accessed 12 January, 2012
- Ajani Jaffer et al. Esophageal and Esophagogastric Junction Cancers. J Nati Compr Cancer Netw. 2011;9:880-887
- Baquet CR, Commiskey P, Mack K, Meltzer S, Mishra SI. Esophageal cancer epidemiology in blacks and whites: racial and gender disparities in incidence, mortality, survival rates and histology. J Natl Med Assoc. 2005 Nov;97(11):1471-8. This article on PubMed
- Gatei DG, et al. Retrospective Study of Carcinoma of the Esophagus in Kenya. Cancer Res. 1978;38:303-307. This article on PubMed
- Krain LS. Esophageal cancer in California 1942-1969: the California Tumor Registry experience. J Surg Oncol. 1973;5(3):267-75. This article on PubMed
- Pedram A, Mahmodlou R, Enshayi A, Sepehrvand N. Esophageal cancer in northwestern Iran. Indian J Cancer. 2011 Apr-Jun;48(2):165-9. This article on PubMed
- Kachala Robson. Systematic review: Epidermiology of Oesophageal Cancer in SubSaharan Africa. Malawi Med J. 2010;22(3):65-70. This article on PubMed
- Dawsey SP, Tonui S, Parker RK et al. Esophageal Cancer in Young People: A case series of 109 cases and review of the literature. PLoS One. 2010 Nov 22;5(11):e14080. This article on PubMed
- Nordenstedt H, El-Serag H. The influence of age, sex, and race on the incidence of esophageal cancer in the United States (1992-2006). Scand J Gastroenterol. 2011 May;46(5):597-602. This article on PubMed
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005 Mar-Apr;55(2):74-108. This article on PubMed
- Rutegard Martin. Oesopageal Cancer: On Surgery and Aetiology. www.publicationskise/jspui/bitstream/10616/39392/1/thesis.pdf..Accessed 12th January, 2012
- Cherian J V, Sivavaman R, Muthusamy AK, Javanthi V. Carcinoma of the esophagus in Tamil Nadu (South India): 16 ? year trends from a tertiary center. J Gastrointestin Liver Dis. 2007;16(3):245-249. This article on PubMed
- Brown LM, Hoover R et al. Excess incidence of squamous cell esophageal cancer among US Black men: role of social class and other risk factors. Am J Epidemiol. 2001 Jan 15;153(2):114-22. This article on PubMed
- Herbella Fernado, Harris Jules., Esophageal Cancer. www.emedicine.medscape.com/article/277930-overview. Accessed 12th January, 2012
- Wikipedia. ESOPHAGEAL CANCER. www.enwikipedia.org/wiki/Esophageal_cancer. Accessed 12 January, 2012
- National Cancer Institute. ESOPHAGEAL CANCER TREATMENT. www.cancer.gov/cancertopics/pdq/treatment/esopageal/HealthProfessional..Accessed 12th January, 2012
- Kollarova Helena, et al. Epidermiology of Esophageal Cancer ? An Overview Article. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2007;151(1):17-28. This article on PubMed
- Chou James, Gress Frank. Esophageal Cancer. www.health.am/cr/esophageal-cancer/ Accessed 13th January, 2012
- Lagergren Jasper, Lagergren Pernilla. Oesophageal Cancer. BMJ. 2010;341:c6280. This article on PubMed
- The Free Dictionary. ESOPHAGEAL CANCER. www.medical-dictionary.thefreedictionary.com/Esophageal+carcinoma. Accessed 13th January, 2012
- Patel AN, Preskitt JT et al. Surgical management of esophageal carcinoma. Proc (Bayl Univ Med Cent). 2003 Jul;16(3):280-4. This article on PubMed
- Lutetich James et al. Evaluation of distant metastasis in esophageal cancer: 100 consercutive positron emission tomography scans. Ann Thorac Surg. 1999;68:1133-1136. This article on PubMed
- Heeren Pierre et al. Detection of Distant Metastasis on Esophageal Cancer with 18F-FDG PET. J Nucl Med. 2004;45(6):980-987. This article on PubMed
- The Society For Surgery of the Alimentary Tract. SURGICAL TREATMENT OF ESOPHAGEAL CANCER. www.ssat.com/cgi-bin/esoph.cgi. Accessed 16th January, 2012