Epidemiology of intussusception in infants less than one year of age in Ghana, 2012-2016
Glover Addy, Daniel Ansong, Christabel Enweronu-Laryea, Jacqueline E Tate, Kwame Amponsa-Achiano, Badu Sarkodie, Jason M Mwenda, StanleyDiamenu, Sandra Kwarteng Owusu, Boateng Nimako, Nicholas Karikari Mensah, Joseph Armachie, Clement Narh, Kimberly Pringle, Scott P Grytdal, Fred Binka, Ben Lopman, Umesh D Parashar, George Armah
Corresponding author: George Armah, Noguchi Medical Institute for Medical Research, University of Ghana, Accra, Ghana 
Received: 10 Aug 2020 - Accepted: 15 Oct 2020 - Published: 29 Jul 2021
Domain: Epidemiology,Immunization
Keywords: Epidemiology, intussusception, Ghana
This article is published as part of the supplement Intussusception in African Infants, commissioned by Supported by the Gavi Alliance through the CDC Foundation.
©Glover Addy et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Glover Addy et al. Epidemiology of intussusception in infants less than one year of age in Ghana, 2012-2016. Pan African Medical Journal. 2021;39(1):8. [doi: 10.11604/pamj.supp.2021.39.1.25445]
Available online at: https://www.panafrican-med-journal.com//content/series/39/1/8/full
Epidemiology of intussusception in infants less than one year of age in Ghana, 2012-2016
Hope Glover-Addy1, ![]() Daniel Ansong2, Christabel Enweronu-Laryea3, Jacqueline E. Tate4, Kwame Amponsa-Achiano5, Badu Sarkodie5, Jason M Mwenda6, Stanley Diamenu6, Sandra Kwarteng Owusu2, Boateng Nimako2,
Daniel Ansong2, Christabel Enweronu-Laryea3, Jacqueline E. Tate4, Kwame Amponsa-Achiano5, Badu Sarkodie5, Jason M Mwenda6, Stanley Diamenu6, Sandra Kwarteng Owusu2, Boateng Nimako2, ![]() Nicholas Karikari Mensah2, Joseph Armachie7,
Nicholas Karikari Mensah2, Joseph Armachie7, ![]() Clement Narh8, Kimberly Pringle4, Scott P Grytdal4,
Clement Narh8, Kimberly Pringle4, Scott P Grytdal4, ![]() Fred Binka8, Ben Lopman9,
Fred Binka8, Ben Lopman9, ![]() Umesh D Parashar4, George Armah7,&
Umesh D Parashar4, George Armah7,&
&Corresponding author
Introduction: we examined the epidemiology, clinical and demographic characteristics of intussusception in Ghanaian infants.
Methods: active sentinel surveillance for pediatric intussusception was conducted at Komfo Anokye Teaching Hospital in Kumasi and Korle Bu Teaching Hospital in Accra. From March 2012 to December 2016, infants < 1 year of age who met the Brighton Collaboration level 1 diagnostic criteria for intussusception were enrolled. Data were collected through parental interviews and medical records abstraction.
Results: a total of 378 children < 1 year of age were enrolled. Median age at onset of intussusception was 27 weeks; only 12 cases (1%) occurred in infants < 12 weeks while most occurred in infants aged 22-34 weeks. Median time from symptom onset until referral to a tertiary hospital was 2 days (IQR: 1-4 days). Overall, 35% of infants were treated by enema, 33% had surgical reduction and 32% required surgical reduction and bowel resection. Median length of hospital stay was 5 days (IQR: 3-8 days) with most patients (95%) discharged home. Eleven (3%) infants died. Infants undergoing enema reduction were more likely than those treated surgically to present for treatment sooner after symptom onset (median 1 vs 3 days; p < 0.0001) and have shorter hospital stays (median 3 vs 7 days; p < 0.001).
Conclusion: Ghanaian infants had a relatively low case fatality rate due to intussusception, with a substantial proportion of cases treated non-surgically. Early presentation for treatment, possibly enhanced by community-based health education programs and health information from various media platforms during the study period might contribute to both the low fatality rate and high number of successful non-surgical treatments in this population.
Intussusception, a condition in which a segment of the intestine invaginates into the adjourning intestinal lumen, is the most common cause of bowel obstruction in young children. The global incidence of intussusception in infants is 74 per 100,000 and varies considerably by region.1 In Africa, few incidence estimates are available; one study estimated a rate of 56 per 100,000 infants < 1 year of age in South Africa [1, 2]. The overall case fatality rate for intussusception cases is 9% in the African region but rates have ranged as high as 28-34% in some countries [1, 3, 4]. The cause of most intussusception cases is not known but some infectious agents, particularly respiratory adenoviruses, have been associated with intussusception [5, 7]. If treatment is sought early, intussusception can be reduced by enema. If treatment is sought late or if facilities with enema reduction capabilities are not available, surgical reduction is required. Intussusception can be fatal if untreated.
In some high- and middle-income settings rotavirus vaccines have been associated with a small, increased risk of intussusception after the first dose of vaccine [8-14]. Subsequently, the African Intussusception Surveillance Network was established in 2012 in 7 sub-Saharan African countries including Ghana to examine the risk of intussusception following monovalent rotavirus vaccination in this region [15]. No increased risk of intussusception following rotavirus vaccination was observed in a pooled analysis of data from these 7 countries or in a similar analysis in South Africa [15, 16]. However, descriptions of the epidemiology of intussusception in sub-Saharan Africa remain limited. This paper seeks to describe the clinical and demographic characteristics of the intussusception cases enrolled in Ghana as part of the African Intussusception Surveillance Network.
Active sentinel surveillance for intussusception was conducted at two large tertiary care hospitals in Ghana. Komfo Anokye Teaching Hospital (KATH), Kumasi, is a 1200 bed hospital in the middle belt of the country; it has 25 pediatric surgical beds and enrolled patients from March 2012 to December 2016. Korle Bu Teaching Hospital (KBTH), Accra, is a 2000 bed facility in the southern belt; the40-bedspediatric surgical unit enrolled patients from September 2013 to December 2016.
Infants < 1 years of age meeting the Brighton Collaboration criteria for level 1 of diagnostic certainty for intussusception [17] were enrolled. To meet Level 1 of diagnostic certainty, the intussusception must be confirmed during surgery, by specific radiologic findings (if reduced by enema), or at autopsy. A case report form collecting limited demographic and treatment information was completed for each case by review of the infant´s medical record and through interview of the infant´s parents or guardians. Descriptive statistics including frequencies and medians were used to summarize the data. Wilcoxon rank-sum tests were used to compare for continuous variables and χ2 tests to compare categorical variables. A p-value of < 0.05 was considered statistically significant.
Disclaimer: the findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC) or the World Health Organization.
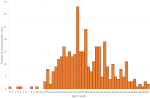
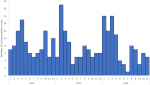
A total of 378 intussusception cases in children < 1 year of age that met the Brighton Collaboration criteria for level 1 of diagnostic certainty for intussusceptions were enrolled from the two surveillance sites. KATH enrolled 155 (41%) infants and KBTH enrolled 223 (59%) infants (Table 1). Just over half (53%) of infants were male. The median age at intussusception onset was 27 weeks. Intussusception was rare in young infants with only 12 cases (1%) occurring in infants < 12 weeks of age. Intussusception cases peaked in infants 22 to 34 weeks of age before gradually declining in older infants (Figure 1). Cases occurred throughout the year with an increase in the number of cases enrolled during January to April of each year (Figure 2).
For most (94%) patients, clinicians recognized clinical symptoms of intussusception when making their diagnosis (Table 2). More than two-thirds of cases (68%) had an ultrasound preformed. At least 130 infants were transferred from another facility to one of the two surveillance hospitals for treatment with a median of 1 day (interquartile range (IQR): 0-2 days) between admission at the first facility and the surveillance facility. The median time from symptom onset until admission at the surveillance facility was 2 days (IQR: 1-4 days) with 72 (19%) infants presenting on the same day as symptom onset and 80 (21%) presenting on the following day. Just over one third (35%) of the infants were treated by enema and the remaining infants were treated surgically. Of those treated surgically, approximately half (49%) required resection of the bowel. The median length of hospital stay was 5 days (IQR: 3-8 days) with most (95%) patients discharged home. Eleven (3%) intussusception patients died. No differences in age or sex were observed between infants undergoing enema reduction compared with infants undergoing surgical reduction (Table 3). Infants undergoing enema reduction were more likely to present to the surveillance facility for treatment sooner after symptom onset (median 1 day (IQR: 0-3)) than infants undergoing surgical reduction (median 3 days (IQR: 1-4); p < 0.0001). Similarly, infants with enema reduction had shorter hospital stays (median 3 days (IQR: 2-4)) than infants with surgical reduction (median 7 days (IQR: 5-10); p < 0.001). No infants with enema reduction died; versus 8 (4%) infants with surgical reduction.
More than one third of intussusception cases were reduced non-surgically at these two large tertiary care hospitals in Ghana. The enema-reduced intussusception cases were more likely to have presented to the surveillance facility for treatment sooner and had short hospital stays than cases that were treated surgically. None of the enema-reduced intussusception cases died. These findings highlight the importance of prompt access to care and availability of non-surgical methods for reduction of intussusception in reducing the morbidity and mortality due to this condition. Intussusception cases that presented late tended to require surgical reduction and had poorer outcomes. Compared with other countries in sub-Saharan Africa where case fatality rates for intussusception often exceed 10% and can be as high as 25%-33%, Ghana´s intussusception case fatality rate of 3% in this evaluation is starkly lower and reflects this access to care and non-surgical reduction methods [1, 3, 4, 18-20]. Additionally, there was a concerted effort to educate health workers and the public about intussusception following the introduction of rotavirus vaccine. The early referral to tertiary centers may also be a result of this drive to improve health literacy through community-based health education programs and health information from various media platforms. Overall, intussusception cases in Ghana presented for care 2 days after symptom onset compared with > 3 days as has been observed in many African countries [21].
Another key finding is that intussusception cases were rare in the first 3 months of life with only 1% of cases occurring before 12 weeks of age. The age distribution of intussusception cases in Ghana is similar to that from a previous analysis in Ghana as in other countries with relatively few cases in early infancy followed by a peak around 6 months of age before tapering downward in older infancy [1, 22]. Rotavirus vaccine is recommended in Ghana at 6 and 10 weeks of age and on-time vaccination will ensure that the vaccine is administered at a time when the risk of intussusception is naturally low.
This analysis has several limitations. First, this was a secondary analysis of data collected to answer a specific question regarding the association between rotavirus vaccination and intussusception. No association between vaccination and intussusception was observed after either dose of vaccine in the larger analysis pooled across multiple countries [15]. Only a limited number of variables were collected for each case which restricts the clinical and epidemiological description of these cases. However, we were able to identify a few unique, informative factors related to the treatment of intussusception in Ghana. Second, surveillance for intussusception was only conducted at two facilities in Ghana. Both facilities were large teaching hospitals and may not be representative of the care received by all intussusception cases in Ghana. However, they do exemplify the level of care that can be provided with sufficient training and facilities.
In summary, observed case fatality rates from intussusception in Ghana were low compared with other countries in Africa. Prompt presentation and diagnosis of intussusception enable cases of intussusception to be treated non-surgically resulting in shorter hospital stays and thereby reducing the burden on the healthcare system.
What is known about this topic
- Intussusception is the most common cause of bowel obstruction in young children;
- The cause of most intussusception is not known but some infectious agents, particularly respiratory adenoviruses, have been associated with intussusception;
- The global incidence of intussusception in infants varies considerably by region; however, descriptions of the epidemiology of intussusception in sub-Saharan Africa remain very limited.
What this study adds
- Intussusception cases in Ghana rare in the first 3 months of life;
- Case fatality rates from intussusception in Ghana were low compared with other countries in Africa;
- Early presentation for treatment, possibly enhanced by community-based health education programs and health information might have contributed to both the low fatality rate and high number of successful non-surgical treatments reported in this study.
The authors declare no competing interests.
All authors have read and agreed to the final version of this manuscript.
Our sincere thanks to all parents and guardians who allowed their wards to participate in this study. Needs acknowledgment of Gavi funding: supported by the Gavi Alliance through the CDC Foundation.
Table 1: demographic characteristics of children with intussusception at the Korle Bu (KBTH) and Komfo Anokye (KATH) Teaching Hospitals in Ghana, 2012-2016
Table 2: clinical characteristics of children with intussusception at the Korle Bu (KBTH) and Komfo Anokye (KATH) Teaching Hospitals in Ghana, 2012-2016
Table 3:
comparison of intussusception cases undergoing enema reduction versus surgical
reduction, Korle Bu (KBTH) and Komfo Anokye (KATH) Teaching Hospitals, Ghana,
2012-2016
Figure 1: age distribution of infants diagnosed with intussusception, Korle Bu (KBTH) and Komfo Anokye (KATH) Teaching Hospitals, Ghana, 2012-2016
Figure 2: seasonality by month and year of intussusception cases, Korle Bu (KBTH) and Komfo Anokye (KATH) Teaching Hospitals, Ghana, 2012-2016
- Jiang J, Jiang B, Parashar U, Nguyen T, Bines J, Patel MM. Childhood intussusception: a literature review. PLoS One. 2013 Jul 22;8(7):e68482. PubMed | Google Scholar
- Moore SW, Kirsten M, Muller EW, Numanoglu A, Chitnis M, Le Grange E et al. Retrospective surveillance of intussusception in South Africa, 1998-2003. J Infect Dis. 2010 Sep 1;202 Suppl:S156-61. PubMed | Google Scholar
- Mpabalwani EM, Chitambala P, Chibumbya JN, Matapo B, Mutambo H, Mwenda JM et al. Intussusception incidence rates in 9 Zambian hospitals, 2007-2011: prerotavirus vaccine introduction. Pediatr Infect Dis J. 2014 Jan;33 Suppl 1:S94-8. PubMed | Google Scholar
- Ngendahayo E, Bonane A, Ntakiyiruta G, Aline Munyanshongore, Narcisse Muganga, Joel Bikoroti et al. Preparing for safety monitoring after rotavirus vaccine implementation: a retrospective review of intussusception cases among children at a large teaching hospital in Randa, 2009-2012. Pediatr Infect Dis J. 2014 Jan;33 Suppl 1:S99-S103. PubMed | Google Scholar
- Bines JE, Liem NT, Justice FA, Tran Ngoc Son, Carl D Kirkwood, Margaret de Campo et al. Risk factors for intussusception in infants in Vietnam and Australia: adenovirus implicated, but not rotavirus. J Pediatr. 2006 Oct;149(4):452-60. PubMed | Google Scholar
- Minney-Smith CA, Levy A, Hodge M, Peter Jacoby, Simon H Williams, Dale Carcione et al. Intussusception is associated with the detection of adenovirus C, enterovirus B and rotavirus in a rotavirus vaccinated population. J Clin Virol. 2014 Dec;61(4):579-84. PubMed | Google Scholar
- Burnett E, Kabir F, Van Trang N, Ajit Rayamajhi, Syed M Satter, Jie Liu et al. Infectious etiologies of intussusception among children < 2 years old in 4 Asian countries. J Infect Dis. 2020 Apr 7;221(9):1499-1505. PubMed | Google Scholar
- Patel MM, Lopez-Collada VR, Bulhoes MM, Lucia Helena De Oliveira, Aurora Bautista Màrquez, Brendan Flannery et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. 2011 Jun 16;364(24):2283-92. PubMed | Google Scholar
- Carlin JB, Macartney KK, Lee KJ, Helen Quinn E, Jim Buttery, Ruth Lopert et al. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia's National Immunization Program. Clin Infect Dis. 2013 Nov;57(10):1427-34. PubMed | Google Scholar
- Yih WK, Lieu TA, Kulldorff M, David Martin, Cheryl N McMahill-Walraven, Richard Platt et al. Intussusception risk after rotavirus vaccination in U.S. infants. N Engl J Med. 2014 Feb 6;370(6):503-12. PubMed | Google Scholar
- Yung CF, Chan SP, Soh S, Tan A, Thoon KC. Intussusception and Monovalent Rotavirus Vaccination in Singapore: Self-Controlled Case Series and Risk-Benefit Study. J Pediatr. 2015 Jul;167(1):163-8.e1 Epub 2015 Apr 14. PubMed | Google Scholar
- Velazquez FR, Colindres RE, Grajales C, Teresa Hernàndez M, Marìa Guadalupe Mercadillo, Javier Torres et al. Postmarketing surveillance of intussusception following mass introduction of the attenuated human rotavirus vaccine in Mexico. Pediatr Infect Dis J. 2012 Jul;31(7):736-44. PubMed | Google Scholar
- Weintraub ES, Baggs J, Duffy J, Claudia Vellozzi, Edward Belongia A, Stephanie Irving et al. Risk of intussusception after monovalent rotavirus vaccination. N Engl J Med. 2014 Feb 6;370(6):513-9. PubMed | Google Scholar
- Stowe J, Andrews N, Ladhani S, Miller E. The risk of intussusception following monovalent rotavirus vaccination in England: A self-controlled case-series evaluation. Vaccine. 2016 Jul 12;34(32):3684-9. PubMed | Google Scholar
- Tate JE, Mwenda JM, Armah G, Bhavin Jani, Richard Omore, Ayesheshem Ademe et al. Evaluation of Intussusception after Monovalent Rotavirus Vaccination in Africa. N Engl J Med. 2018 Apr 19;378(16):1521-1528. PubMed | Google Scholar
- Groome MJ, Tate JE, Arnold M, Milind Chitnis, Sharon Cox, Corné de Vos et al. Evaluation of intussusception after oral monovalent rotavirus vaccination in South Africa. Clin Infect Dis. 2020 Apr 10;70(8):1606-1612. PubMed | Google Scholar
- Bines JE, Kohl KS, Forster J, Lynn R Zanardi, Robert L Davis, John Hansen et al. Acute intussusception in infants and children as an adverse event following immunization: case definition and guidelines of data collection, analysis, and presentation. Vaccine. 2004 Jan 26;22(5-6):569-74. PubMed | Google Scholar
- Mensah Y, Glover-Addy H, Etwire V, Appeadu-Mensah W, Twum M. Ultrasound guided hydrostatic reduction of intussusception in children at Korle Bu Teaching Hospital: an initial experience. Ghana Med J. 2011 Sep;45(3):128-31. PubMed | Google Scholar
- Mensah YB, Glover-Addy H, Etwire V, Margaret Twum B, Samuel Asiamah, William Appeadu-Mensah et al. Pneumatic reduction of intussusception in children at Korle Bu Teaching Hospital: an initial experience. Afr J Paediatr Surg. May-Aug 2011;8(2):176-81. PubMed | Google Scholar
- Abantanga FA, Amoah M, Adeyinka AO, Nimako B, Yankey KP. Pneumatic reduction of intussusception in children at the Komfo Anokye Hospital, Kumasi, Ghana. East Afr Med J. 2008 Nov;85(11):550-5. PubMed | Google Scholar
- Mpabalwani EM, Mwenda JM, Tate JE, Parashar UD. Review of Naturally Occurring Intussusception in Young Children in the WHO African Region prior to the Era of Rotavirus Vaccine Utilization in the Expanded Programme of Immunization. J Trop Pediatr. 2017 Jun 1;63(3):221-228. PubMed | Google Scholar
- Enweronu-Laryea CC, Sagoe KW, Glover-Addy H, Asmah RH, Mingle JA, Armah GE. Prevalence of severe acute rotavirus gastroenteritis and intussusceptions in Ghanaian children under 5 years of age. J Infect Dev Ctries. 2012 Feb 13;6(2):148-55. PubMed | Google Scholar
Search
This article authors
On Pubmed
- Glover Addy
- Daniel Ansong
- Christabel Enweronu-Laryea
- Jacqueline E Tate
- Kwame Amponsa-Achiano
- Badu Sarkodie
- Jason M Mwenda
- StanleyDiamenu
- Sandra Kwarteng Owusu
- Boateng Nimako
- Nicholas Karikari Mensah
- Joseph Armachie
- Clement Narh
- Kimberly Pringle
- Scott P Grytdal
- Fred Binka
- Ben Lopman
- Umesh D Parashar
- George Armah
On Google Scholar
- Glover Addy
- Daniel Ansong
- Christabel Enweronu-Laryea
- Jacqueline E Tate
- Kwame Amponsa-Achiano
- Badu Sarkodie
- Jason M Mwenda
- StanleyDiamenu
- Sandra Kwarteng Owusu
- Boateng Nimako
- Nicholas Karikari Mensah
- Joseph Armachie
- Clement Narh
- Kimberly Pringle
- Scott P Grytdal
- Fred Binka
- Ben Lopman
- Umesh D Parashar
- George Armah