Immunity, parasites, genetics and sex hormones: contributors to mild inflammatory responses in COVID-19?
Samuel Munalula Munjita, Mulemba Samutela, Kunda Ndashe, Sody Mweetwa Munsaka
Corresponding author: Samuel Munalula Munjita, Department of Biomedical Sciences, School of Health Sciences, University of Zambia, Nationalist Road, Lusaka, Zambia 
Received: 02 May 2020 - Accepted: 12 May 2020 - Published: 15 May 2020
Domain: Immunology,Microbiology,Physiology
Keywords: COVID-19, mild symptoms, cytokines, inflammation, immunity, parasites, genetics, hormones
This article is published as part of the supplement PAMJ Special issue on COVID - 19 in Africa, commissioned by The Pan African Medical Journal.
©Samuel Munalula Munjita et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Samuel Munalula Munjita et al. Immunity, parasites, genetics and sex hormones: contributors to mild inflammatory responses in COVID-19?. Pan African Medical Journal. 2020;35(2):36. [doi: 10.11604/pamj.supp.2020.35.2.23267]
Available online at: https://www.panafrican-med-journal.com/content/series/35/2/36/full
Essay 
Immunity, parasites, genetics and sex hormones: contributors to mild inflammatory responses in COVID-19?
Immunity, parasites, genetics and sex hormones: contributors to mild inflammatory responses in COVID-19?
Samuel Munalula Munjita1,2,&, Mulemba Samutela1,3, Kunda Ndashe4, Sody Mweetwa Munsaka1
1Department of Biomedical Sciences, School of Health Sciences, University of Zambia, Lusaka, Zambia, 2Department of Disease Control, School of Veterinary Medicine, University of Zambia, Lusaka, Zambia, 3Department of Paraclinical studies, School of Veterinary Medicine, University of Zambia, Lusaka, Zambia, 4Department of Environmental Health, Faculty of Health Sciences, Lusaka Apex Medical University, Lusaka, Zambia
&Corresponding author
Samuel Munalula Munjita, Department of Biomedical Sciences, School of Health Sciences, University of Zambia, Nationalist Road, Lusaka, Zambia
The Coronavirus disease 2019 (COVID-19) pandemic has killed over two hundred thousand people by end of April, 2020. America and Europe top in deaths from COVID-19 whereas the numbers are lower in Africa for unclear reasons. Emerging evidence suggests the role of hyperactive immune responses characterised by high pro-inflammatory cytokines in severe cases of COVID-19 and deaths. In this perspective, we explore the possible factors that may contribute to mild inflammatory responses in some cases of COVID-19 by focusing on immune education, parasites, sex hormones and chronic diseases, as well as genetic tolerance. To build our perspective, evidence is also extracted from wild rodents due to their multi-tasking immune responses as a result of constant exposure to pathogens.
Immune responses characterised by high levels of cytokines have been associated with poor prognosis for COVID-19 [1-3]. People with compromised immune responses due to old age and chronic diseases are considered to be at higher risk of the disease [4,5]. Conversely, children (except infants) and teens, often known to have immature immune systems, generally have mild to moderate symptoms for COVID-19 [5,6]. Meanwhile relatively low death rates in some regions with a high infectious disease burden and a relatively young population have been observed [7]. Although it is too early to make meaningful conclusions, immature and poorly functional Angiotensin-converting enzyme II (ACE2) receptors have been suggested to play a role in mild COVID cases among children [8,9]. Regardless, we think that other contributing factors include cross-reactive antibodies from seasonal coronavirus infections, genetic tolerance, sex hormones, and frequent contact with diverse pathogens and antigens. An immune system continuously exposed to pathogens may be educated to respond favourably to avoid immune-mediated pathology as observed elsewhere [10]. At the centre of such a system are mild to moderate cytokine responses. Although not the mainstay of this perspective, we accept that previous vaccination with Bacillus Calmette-Guerin (BCG) may have non-specific protective effects against SARS-CoV-2 infections [11,12]. In this perspective, we analyse the factors and mechanisms that may contribute to mild or asymptomatic cases of SARS-CoV-2 infections. We base some of the assumptions and perspectives on immune responses in wild rodents which constantly encounter various pathogens.
Inflammation in COVID-19: free living rodents in the wild are frequently exposed to various infectious agents. Their immune systems may be experienced to produce aggressive immune responses, but have evolved to respond mildly [10,13]. This may be a homeostatic mechanism to reduce immune-mediated injury [13]. Although this does not apply to all viruses [14-16], lessons can be drawn to understand the possible mechanisms that may lead to asymptomatic COVID-19 or severe cases. Generally, immune responses of rodents in the wild are characterised by highly elevated Th2 responses with depressed Th1 cytokine responses [17,18]. In contrast, laboratory rodents with no frequent contact with pathogens and/or antigens have greater immunological focus resulting in strong Th1 proinflammatory cytokine responses during viral infections [10]. Similarly, severe COVID-19 cases are associated with increase in Th1 and Th17 cell proportions as well as inflammatory CD14+CD16+ monocytes [3,19]. This has been linked to high expression of Interleukin 6 (IL-6) and Interleukin 23 (IL-23) that accelerate inflammation and promote the conversion of naïve CD4+ T cells into Th17 cells [20,21]. Consequently, high levels of Th1, Th17, and inflammatory CD14+CD16+ monocytes release more cytokines that activate macrophages and fibroblasts resulting in overwhelming amounts of proinflammatory cytokines [21,22] associated with severe lung pathology in COVID-19 patients [3].
Oestrogen and testosterone: overwhelming cytokine responses in older patients with COVID-19 follow this mechanism [2,3,19]. However, we think that hyper inflammation in older patients may also be related to pre-existing high levels of IL-6 and other pro-inflammatory cytokines associated with low oestrogen and testosterone levels due to menopause or andropause [23]. Oestrogen and testosterone are known inhibitors of secretion of IL-6 [23] but where low muscle mass (in the case of men) and chronic disease such as diabetes, hypertension, and heart diseases exist, the effects of oestrogen and testosterone are downgraded thereby risking an individual to cytokine storms. Thus, we believe that levels of oestrogen and testosterone may determine an individual´s susceptibility to severe COVID-19 and death. Chronic diseases and obesity do not only suppress oestrogen and testosterone but also promote high levels of pro-inflammatory cytokines making the risk of severe SARS-CoV2 infections even higher. This explains the high cases of severe COVID-19 cases and death rates in Europe and North America due to a high population of old people and those with chronic diseases. Conversely, we expect fewer cases of severe forms of this disease and deaths in most Sub-Saharan African countries where the majority of people are young but high HIV rates may dilute this effect.
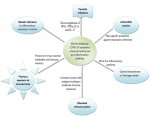
Educated immune systems: since proinflammatory cytokines are at the centre of the patho-mechanism of severe SARS-CoV-2 infections, we think that an educated immune system particurly in immune-competent individuals may have a role in asymptomatic COVID-19 (Figure 1). We define an educated immune system as one that has had frequent exposure to all kinds of pathogens and antigens and is evolved enough to respond without causing self-harm. Such an immune system may be common in immune-competent people living in infectious disease burdened areas and in those exposed to various respiratory pathogens in public places (schools, colleges, Universities, hospitals etc) due to close contact. Similar to what is observed in wild rodents, we expect such individuals to have immune responses to SARS-CoV-2 characterised by a balanced Th1 and Th2 cytokine response that is neither depressed nor hyperactive but none pathological. This is because daily exposure to pathogens allows the immune system to have a much less focused immune response thereby lowering its aggressiveness but maintaining its effectiveness [10,24]. However, where the individual has had exposure to parasitic infections (intestinal helminths and others), the immune responses will be skewed towards elevated Th2 responses with depressed Th1 cytokines resulting in mild immune mediated damage during virus infection such as SARS-CoV-2. Part of our conviction regarding the role of constant contact with pathogens in the environment in moderating COVID-19 progression is based on the predominant theory regarding the rise of allergic reactions brought about by improvements in vaccination and sanitation [25-27]. In both allergic reactions and infectious diseases, it appears that immune education is vital for favourable prognosis of some diseases or infections. In line with exposure theory, we think that previous exposure to some strains of coronaviruses antigenically similar to SARS-CoV-2 may also be a contributing factor to some cases of mild or asymptomatic COVId-19. We assume that cross-reactive antibodies may account for the protection. There is need to evaluate antibody cross reactivity among coronaviruses.
Parasitic infections: these pathogens up-regulate IL-4 and IL-10 cytokines resulting in increased Th2 differentiation and down-regulation of IFNγ, TNFα, IL-6, and IL-17 inflammatory responses, respectively [18,25,28]. We suppose that this immune mechanism is active in wild rodents that presumably have high parasitic infestations and may play a role in trade-off mechanisms between viruses and mice [13,17]. Thus, parasite driven down-regulation of pro-inflammatory mediators suggest the potential of these pathogens to locally and systematically block cytokine storms observed in COVID-19 cases or any other viral illness [25,28]. Taken together, we speculate that such a scenario is possible in parasite infested individuals living in resource-limited communities where regular deworming is not practised. Therefore, we envisage mild to moderate or self-limiting COVID-19 cases in such individuals as long as their immune system is competent and are free of chronic diseases.
Genetic tolerance: mortality and vulnerability data for the COVID-19 infections in China show sex differences between men and women with more men dying than women [29]. Smoking and other risky behaviours among men have been suggested as possible reasons for the observation [29]. Here we suggest the role of genetic tolerance to cytokine storms in women. IL-6, one of the cytokines at the centre of the pathology of COVID-19, is widely expressed in the female reproductive tract and gestational tissues to enable embryo implantation, placenta development, and pregnancy tolerance [30]. We speculate that immuno-competent women may be genetically prepared to handle cytokine storms than men leading lowered risk of COVID-19 associated death.
The mechanism(s) for the occurrence of mild or severe COVID-19 is related to an individual´s immune system, particularly the levels of pro-inflammatory responses. Genetic tolerance related to pregnancy may also have a role to play. Parasites, sex hormones, and constant exposure to pathogens may help lower the risk of pathological immune responses in SARS CoV-2 infections in immuno-competent individuals. Since the risk of severe disease is high among the elderly and those with chronic diseases, community testing for the virus must prioritise these groups of people in the light of shortage of testing kits. This will ensure that those found positive receive appropriate medical attention before they show any symptoms.
The authors declare no competing interests.
Conceptualisation: Samuel Munalula Munjita; formal analysis: Samuel Munalula Munjita, Mulemba Samutela, Sody Mweetwa Munsaka; writing, original draft preparation: Samuel Munalula Munjita; writing, review and editing: Samuel Munalula Munjita, Mulemba Samutela, Kunda Ndashe, Sody Mweetwa Munsaka. All authors have read and agreed to the final version of the manuscript.
The authors thank the African Centre of Excellence for Infectious Diseases in Humans and Animals (ACEIDHA) at the University of Zambia, School of Veterinary Medicine who are the PhD sponsors for Samuel Munalula Munjita and Mulemba Samutela.
Figure 1: factors and mechanisms that may contribute to mild inflammatory responses in COVID-19 leading to mild-moderate or asymptomatic disease
- Yang Y, Shen C, Li J, Yuan J, Yang M, Wang F et al. Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome. medRxiv. 2020. Google Scholar
- Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020 Mar 29:105954. PubMed | Google Scholar
- Zhou Y, Fu B, Zheng X, Wang D, Zhao C. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. 2020 Mar 13: nwaa041. PubMed
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507-13. PubMed | Google Scholar
- Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020. Google Scholar
- Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020 Mar 25. pii: S1473-3099(20)30198-5. PubMed | Google Scholar
- Price M. Is the South African Covid-19 epidemic different from the rest of the world? Daily Maverick. Accessed 27th April, 2020.
- Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450-4. PubMed | Google Scholar
- Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260-3. PubMed | Google Scholar
- Abolins S, King EC, Lazarou L, Weldon L, Hughes L, Drescher P et al. The comparative immunology of wild and laboratory mice, Mus musculus domesticus. Nat Commun. 2017 May 3;8:14811. PubMed | Google Scholar
- Kristensen I, Fine P, Aaby P, Jensen H. Routine vaccinations and child survival: follow up study in Guinea-Bissau, West AfricaCommentary: an unexpected finding that needs confirmation or rejection. BMJ. 2000 Dec 9;321(7274):1435-8. PubMed | Google Scholar
- Miller A, Reandelar MJ, Fasciglione K, Roumenova V, Li Y, Otazu GH. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. medRxiv. 2020. Google Scholar
- Alizon S, Hurford A, Mideo N, Van Baalen M. Virulence evolution and the trade-off hypothesis: history, current state of affairs and the future. J Evol Biol. 2009 Feb;22(2):245-59. PubMed | Google Scholar
- Mariën J, Sluydts V, Borremans B, Gryseels S, Broecke BV, Sabuni CA et al. Arenavirus infection correlates with lower survival of its natural rodent host in a long-term capture-mark-recapture study. Parasit Vectors. 2018 Feb 8;11(1):90. PubMed | Google Scholar
- Vitullo AD, Hodara VL, Merani MS. Effect of persistent infection with Junin virus on growth and reproduction of its natural reservoir, Calomys musculinus. Am J Trop Med Hyg. 1987 Nov;37(3):663-9. PubMed | Google Scholar
- Webb P, Justines G, Johnson K. Infection of wild and laboratory animals with Machupo and Latino viruses. Bull World Health Organ. 1975;52(4-6):493-9. PubMed | Google Scholar
- Moreau E, Chauvin A. Immunity against helminths: interactions with the host and the intercurrent infections. J Biomed Biotechnol. 2010;2010:428593. PubMed | Google Scholar
- Moreels T, Nieuwendijk R, De Man J, Winter D, Herman A, Van Marck E et al. Concurrent infection with Schistosoma mansoni attenuates inflammation induced changes in colonic morphology, cytokine levels, and smooth muscle contractility of trinitrobenzene sulphonic acid induced colitis in rats. Gut. 2004;53(1):99-107. PubMed | Google Scholar
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet respiratory medicine. 2020;8(4):420-2. PubMed | Google Scholar
- Miossec P, Kolls JK. Targeting IL-17 and TH 17 cells in chronic inflammation. Nat Rev Drug Discov. 2012 Oct;11(10):763-76. PubMed | Google Scholar
- Romagnani S, Maggi E, Liotta F, Cosmi L, Annunziato F. Properties and origin of human Th17 cells. Mol Immunol. 2009 Nov;47(1):3-7. PubMed | Google Scholar
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M et al. Reciprocal developmental pathways for the generation of pathogenic effector TH 17 and regulatory T cells. Nature. 2006;441(7090):235-8. PubMed | Google Scholar
- Naugler WE, Karin M. The wolf in sheep's clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends in molecular medicine. 2008;14(3):109-19. Google Scholar
- Brockman MA, Kwon DS, Tighe DP, Pavlik DF, Rosato PC, Sela J, et al. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood. 2009;114(2):346-56. PubMed | Google Scholar
- Bashir MEH, Andersen P, Fuss IJ, Shi HN, Nagler-Anderson C. An enteric helminth infection protects against an allergic response to dietary antigen. J Immunol. 2002 Sep 15;169(6):3284-92. PubMed | Google Scholar
- Epidemiology NCPER. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020 Feb 10;41(2):145-151. PubMed | Google Scholar
- Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989 Nov 18;299(6710):1259-60. PubMed | Google Scholar
- Osada Y, Shimizu S, Kumagai T, Yamada S, Kanazawa T. Schistosoma mansoni infection reduces severity of collagen-induced arthritis via down-regulation of pro-inflammatory mediators. Int J Parasitol. 2009 Mar;39(4):457-64. PubMed | Google Scholar
- Wenham C, Smith J, Morgan R. COVID-19: the gendered impacts of the outbreak. The Lancet. 2020;395(10227):846-8. PubMed | Google Scholar
- Jasper MJ, Tremellen KP, Robertson SA. Reduced expression of IL-6 and IL-1γ mRNAs in secretory phase endometrium of women with recurrent miscarriage. J Reprod Immunol. 2007 Feb;73(1):74-84. Epub 2006 Oct 10.. PubMed | Google Scholar
Search
This article authors
On Pubmed
On Google Scholar
Citation [Download]
Navigate this article
Similar articles in
Key words
Tables and figures
This supplement
- Clinical presentation, case management and outcomes for the first 32 COVID-19 patients in Nigeria (Accessed 18958 times)
- COVID-19 and the Nigerian child: the time to act is now (Accessed 17894 times)
- Profil clinique, biologique et radiologique des patients Algériens hospitalisés pour COVID-19: données préliminaires (Accessed 10355 times)
- The COVID-19 pandemic and social distancing in Nigeria: ignorance or defiance (Accessed 6430 times)
- Knowledge, risk perception and preparedness towards coronavirus disease-2019 (COVID-19) outbreak among Ghanaians: a quick online cross-sectional survey (Accessed 6234 times)
- Continuity of health service delivery during the COVID-19 pandemic: the role of digital health technologies in Uganda (Accessed 4205 times)
- Knowledge, risk perception and preparedness towards coronavirus disease-2019 (COVID-19) outbreak among Ghanaians: a quick online cross-sectional survey (Downloaded 868 times)
- Clinical presentation, case management and outcomes for the first 32 COVID-19 patients in Nigeria (Downloaded 611 times)
- The COVID-19 pandemic and social distancing in Nigeria: ignorance or defiance (Downloaded 601 times)
- Profil clinique, biologique et radiologique des patients Algériens hospitalisés pour COVID-19: données préliminaires (Downloaded 473 times)
- Continuity of health service delivery during the COVID-19 pandemic: the role of digital health technologies in Uganda (Downloaded 456 times)
- COVID-19 and the Nigerian child: the time to act is now (Downloaded 350 times)