Unusual association of COVID-19, pulmonary tuberculosis and human immunodeficiency virus, having progressed favorably under treatment with chloroquine and rifampin
Fah Bouaré, Mehdi Laghmari, Felicité Nyafame Etouche, Badr Arjdal, Imane Saidi, Farouk Hajhouji, Houssine Ghannane, Lamyae Amro, Noura Tassi, Said Ait Benali
Corresponding author: Fah Bouaré, Coronavirus Infection Unit, Department of Neurosurgery of The Arrazi Hospital, King Mohammed VI University Teaching Hospital, BP2360 Principal, Ibn Sina Avenue, Marrakesh 
Received: 12 Jul 2020 - Accepted: 12 Jul 2020 - Published: 13 Jul 2020
Domain: Emergency medicine,Infectious disease,Health Research
Keywords: Coronavirus, tuberculosis, HIV, COVID-19, anti-tubercular agents, rifampin, SARS-ncov-2
This article is published as part of the supplement PAMJ Special issue on COVID - 19 in Africa, commissioned by The Pan African Medical Journal.
©Fah Bouaré et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Fah Bouaré et al. Unusual association of COVID-19, pulmonary tuberculosis and human immunodeficiency virus, having progressed favorably under treatment with chloroquine and rifampin. Pan African Medical Journal. 2020;35(2):110. [doi: 10.11604/pamj.supp.2020.35.2.24952]
Available online at: https://www.panafrican-med-journal.com//content/series/35/2/110/full
Case report 
Unusual association of COVID-19, pulmonary tuberculosis and human immunodeficiency virus, having progressed favorably under treatment with chloroquine and rifampin
Unusual association of COVID-19, pulmonary tuberculosis and human immunodeficiency virus, having progressed favorably under treatment with chloroquine and rifampin
Fah Bouaré1,&, Mehdi Laghmari1, Felicité Nyafame Etouche2, Badr Arjdal1, Imane Saidi3, Farouk Hajhouji1, Houssine Ghannane1, Lamyae Amro3, Noura Tassi2, Said Ait Benali1
&Corresponding author
Infection with the new coronavirus has been declared an international health emergency. Its curative treatment is unknown and is the subject of several clinical trials. In addition, the concomitant association of COVID-19 with tuberculosis and the human immunodeficiency virus, hitherto never described, is potentially fatal. We report the illustrative case of a 32-year-old patient who presented this trifecta of infections and who did well under treatment with chloroquine and anti-mycobacterial drugs. This patient arrived at the ER with respiratory discomfort that had been evolving over a month with symptoms of flu and deterioration of her general condition. A chest CT scan revealed an aspect of lung miliary tuberculosis with isolation of Koch's bacilli in the sputum. A polymerization chain reaction (PCR) was positive for COVID-19 on a nasopharyngeal swab. HIV serology was positive. The course was marked by a spectacular clinical improvement and two negative COVID-19 PCR controls at the end of treatment (at days 9 and 10). Anti-tubercular drugs (especially, rifampin) are powerful enzyme inducers that can reduce the effectiveness of chloroquine in our patient. This therapeutic success may be linked to the effect of anti-tubercular drugs against SARS ncov-2, especially rifampin, inhibiting the formation of messenger RNAs of SARS ncov-2 or to the synergistic effect of chloroquine and rifampin. Researchers should explore the effect of these drugs on SARS ncov-2.
The new coronavirus disease 2019 (COVID-19) may cause severe acute respiratory syndrome (SARS) [1,2]. It is an infection affecting humans, which started in China, in the region of Wuhan, in the province of Hubei. The infection has been declared as an international health emergency [3]. Its curative treatment is unknown, and is the subject of several clinical trials on several types of molecules. We report the illustrative case of a patient who presents this rare combination of poly-infection (tuberculosis, human immunodeficiency virus and COVID-19) and who has progressed well with treatment with chloroquine and anti-tubercular drugs. In the light of this singular observation, we will propose new theories of the action of rifampin on COVID-19 and of the synergistic anti-retroviral and immunomodulatory association of the association of chloroquine with rifampin.
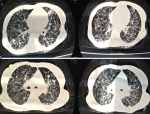
We report a rare case of a poly-infection (with COVID-19) with a very good course of the COVID-19 infection under chloroquine and rifampicin. The patient was a 32-year-old female, with no peculiar medical history, who presented to the ER with respiratory discomfort which had progressed for a month with influenza-like symptoms (feeling of fever, cough, headache, myalgia). She was feverish at 38.1 degrees celsius, had polypnea at 23 cycles per minute, normal heart rate at 80 beats per minute with an arterial pressure at 95/70 millimeters of mercury, her weight was 50 kilogram for a height of 1.60 meter. She had edema on her lower limbs and stage 1 gluteal ulcers. Upon suspecting COVID-19 infection in the patient, lab and imaging studies were carried out: A Polymerization Chain Reaction was positive for COVID-19 on a nasopharyngeal sample; A thoracic computed tomography (Figure 1) was able to identify multiple micronodules very likely related to a miliary tuberculosis. The bacteriological study of 3 sputum samples showed an infection with Koch´s bacillus. Human immunodeficiency virus (HIV) serology was positive with a low CD4 T lymphocytes count of 32 elements/microliter. The patient had anemia with 7.6 gram/deciliter hemoglobin, thrombocytopenia of 70,000 elements/microliter, leucopenia of 2,880 elements/microliter, hyper-ferritinemia at 8,972 nanogram/milliliter.
The diagnosis of lung miliary tuberculosis with COVID-19 infection was evoked, in a setting of retroviral immunosuppression with HIV. The patient was put on anti-tubercular treatment at the daily dose adapted to her weight (isoniazid 5 milligram/kilogram/day, rifampin 10 milligram/kilogram/day, pyrazinamide 30 milligram/kilogram/day and ethambutol 25 milligram/kilogram/day). For the treatment of COVID-19, the patient was put on chloroquine (500 milligram x 2/day) for 10 days and azithromycin (500 milligram the first day then 250 milligram/day for 7 days). The progression was marked by the obtaining of two negative PCR controls at the end of treatment (on days 9 and 10), defervescence of the fever, reduction in respiratory discomfort and cough. Currently, after a follow-up of one and a half months, she continues to improve under anti-mycobacterial treatment, with a weight of 53 kilogram and good cicatrization of the gluteal ulcers.
At this time of the corona virus pandemic there is no consensus on the ideal therapy for this infection [4,5]. Therapeutic trials, in the clinical trials register of the World Health Organization (WHO), have been conducted on the efficacy of certain molecules including chloroquine [6]. Chloroquine is an anti-malarial molecule which acts by the accumulation of heme of hemoglobin, which is toxic to the parasite which causes malaria [7] and an anti-rheumatic treatment [8]. It was administered to the patient at a standard dose and duration recommended for all our COVID-19 patients, because of its effectiveness on the coronavirus by inhibiting its proliferation in-vitro [9,10]. For lack of effective treatment proven by studies with a high level of evidence, chloroquine is adopted in empirical practice in a majority of centers worldwide. In addition, the treatment of tuberculosis is based on the use of anti-tubercular drugs [4,7,11]. Pulmonary tuberculosis patients benefit from an initial treatment of two months combining: isoniazid, rifampin, pyrazinamide and ethambutol [2]. These are molecules with intracellular and or extracellular actions acting on Koch´s bacillus. Rifampin is bactericidal by inhibiting RNA polymerase during the transcription of bacterial deoxyribonucleic acid (DNA) into messenger ribonucleic acid (RNA) [7].
In addition, HIV infection causes immunodepression which is a risk factor for severe forms of coronavirus infection according to the literature [4]. Anti-tubercular drugs (especially, rifampin) are powerful enzyme inducers [2,4,8] that can reduce the effectiveness of chloroquine in our patient. And immunodepression induced by HIV infection can make COVID-19 infection severe. The success of the treatment which resulted in obtaining 2 negative polymerization chain reaction COVID-19 tests, and the improvement of clinical signs, may be linked to an effect of the anti-tubercular drugs against the SARS-cov-2 infection or to the synergistic anti-retroviral effect of the chloroquine-rifampin combination. The action of rifampin, inhibiting the formation of messenger RNAs, should put researchers on the track of its anti-retroviral efficacy on COVID-19 by inhibiting or blocking the synthesis of viral proteins, as well as the track of the synergistic anti-retroviral and immunomodulatory effect of the chloroquine-rifampin combination. Large-scale, randomized studies are needed to verify these hypotheses.
The new coronavirus 2019 infection is currently a real public health problem worldwide. Its treatment is not yet well codified and consensual. The action of rifampin, which inhibits the formation of messenger RNAs, transfer RNAs and ribosomal RNAs, should put researchers on the track of the antiviral efficacy of anti-tubercular drugs or of rifampin on COVID-19. And possibly, that of the synergistic chloroquine-rifampin association could be explored by large-scale, randomized studies, to verify these hypotheses.
The authors declare no competing interests.
Each author contributed to the writing of this manuscript, either by the correction, the reading, or the translation into English. All the authors have read and agreed to the final manuscript.
Figure 1: a thoracic computed tomography without injection of contrast product showing multiple micronodules, very likely in relation to a miliary tuberculosis, without typical signs of COVID-19
- Organisation mondiale de la santé. Centre de traitement des infections respiratoires aiguës sévères. Manuel pratique pour la mise en place et la gestion d´un centre de traitement des IRAS et d´une unité de dépistage des IRAS dans les établissements de soins. Accessed April 24, 2020.
- Ramrakha Punit, Moore Kevin, Sam Amir. Oxford handbook of acute medicine. 4th Ed. New york: oxford university press; 2019.
- Guan Wei-jie, Ni Zheng-yi, Hu Yu, Liang W, Chun-quan Ou, He J et al. Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med. 2020. Google Scholar
- Papadakis Maxine A, McPhee Stephen J, Rabow Michael W. Current medical diagnosis and treatment. 20th Ed. New york: McGraw-hill; 2020.
- Organisation mondiale de la santé. Prise en charge clinique de l´infection respiratoire aiguë sévère lorsqu´une infection par le nouveau coronavirus (2019-nCoV) est soupçonnée. Accessed April 24, 2020.
- Baden Lindsey R , Rubin Eric J. Covid-19-The Search for Effective Therapy. N Engl J Med. 2020 May 7;382(19):1851-1852. PubMed | Google Scholar
- Katzung Bertram G, Kruidering-Hall Marieke, Trevor Anthony J. Katzung and Trevor´spharmacology. 12th Ed. New york: McGraw-Hill Education; 2019.
- Zammitt Nicolas, O´Brien Alastair, Kumar Parveen, Clark Michael. Essentials of Kumar and Clark´s clinical medicine. 6th Ed. China: Elsevier; 2018.
- Lecuit M. Chloroquine and COVID-19, where do we stand? Med Mal Infect. 2020 May; 50(3): 229-230. PubMed | Google Scholar
- Satyajit T, Barsha D, Somenath R, Hlupheka C, Gilbert MM. A review on possible modes of action of chloroquine/hydroxychloroquine: repurposing against SAR-CoV-2 (COVID-19) pandemic. Int J Antimicrob Agents. 2020 May 22;106028. PubMed | Google Scholar
- Mehdi L, Gedéon T, Davis M, Said AB. Tuberculosis of the Nervous System in Immunocompromised Hosts. Tuberculosis of the Central Nervous System. Switzerland: Springer. 2017;499-509. Google Scholar
Search
This article authors
On Pubmed
On Google Scholar
Citation [Download]
Navigate this article
Similar articles in
Key words
Tables and figures
This supplement
- Clinical presentation, case management and outcomes for the first 32 COVID-19 patients in Nigeria (Accessed 18958 times)
- COVID-19 and the Nigerian child: the time to act is now (Accessed 17894 times)
- Profil clinique, biologique et radiologique des patients Algériens hospitalisés pour COVID-19: données préliminaires (Accessed 10355 times)
- The COVID-19 pandemic and social distancing in Nigeria: ignorance or defiance (Accessed 6430 times)
- Knowledge, risk perception and preparedness towards coronavirus disease-2019 (COVID-19) outbreak among Ghanaians: a quick online cross-sectional survey (Accessed 6234 times)
- Continuity of health service delivery during the COVID-19 pandemic: the role of digital health technologies in Uganda (Accessed 4205 times)
- Knowledge, risk perception and preparedness towards coronavirus disease-2019 (COVID-19) outbreak among Ghanaians: a quick online cross-sectional survey (Downloaded 868 times)
- Clinical presentation, case management and outcomes for the first 32 COVID-19 patients in Nigeria (Downloaded 611 times)
- The COVID-19 pandemic and social distancing in Nigeria: ignorance or defiance (Downloaded 601 times)
- Profil clinique, biologique et radiologique des patients Algériens hospitalisés pour COVID-19: données préliminaires (Downloaded 473 times)
- Continuity of health service delivery during the COVID-19 pandemic: the role of digital health technologies in Uganda (Downloaded 456 times)
- COVID-19 and the Nigerian child: the time to act is now (Downloaded 350 times)