A case report: 46,XX Congenital Adrenal Hyperplasia (CAH)
I Gusti Lanang Andi Suharibawa, Besut Daryanto
Corresponding author: I Gusti Lanang Andi Suharibawa, Department of Urology, Faculty of Medicine, Brawijaya University, Saiful Anwar General Hospital, Malang, East Java, Indonesia 
Received: 30 Mar 2018 - Accepted: 16 Jul 2018 - Published: 08 Nov 2018
Domain: Urology
Keywords: 46,XX, congenital adrenal hyperplasia, steroidogenesis
This article is published as part of the supplement Malang Continuing Urology Education: Reconstruction and Functional Urology, commissioned by Committee for the 1st InaGURS International Workshop & Symposium of Reconstruction &.
©I Gusti Lanang Andi Suharibawa et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: I Gusti Lanang Andi Suharibawa et al. A case report: 46,XX Congenital Adrenal Hyperplasia (CAH). Pan African Medical Journal. 2018;31(1):9. [doi: 10.11604/pamj.supp.2018.31.1.15621]
Available online at: https://www.panafrican-med-journal.com//content/series/31/1/9/full
A case report: 46,XX Congenital Adrenal Hyperplasia (CAH)
I Gusti Lanang Andi Suharibawa1,&, Besut Daryanto1
1Department of Urology, Faculty of Medicine, Brawijaya University, Saiful Anwar General Hospital, Malang, East Java, Indonesia
&Corresponding author
I Gusti Lanang Andi Suharibawa, Department of Urology, Faculty of Medicine, Brawijaya University, Saiful Anwar General Hospital, Malang, East Java, Indonesia
Congenital Adrenal Hyperplasia (CAH) are any of several autosomal recessive diseases resulting from mutations of genes for enzymes mediating the biochemical steps of production of mineralocorticoids, glucocorticoids or sex steroids from cholesterol by the adrenal glands (steroidogenesis). This study present A case report of a 15-year-old child with 46,XX congenital adrenal hyperplasia (CAH). 46,XX CAH is a rare case. Management of the patient is based on substantial examination, and the patient's preference has an important role in deciding the type of treatment.
Congenital adrenal hyperplasia (CAH) is a group of autosomal recessive disorders characterized by a defect in one of five enzymes involved in the synthesis of cortisol, aldosterone or both [1]. CAH is also the most common disorder of sex development [2]. It occurs with an incidence ranging from 1 in 5000 to 1 in 15,000 in the United States and Europe. Mutations or partial deletions that affect CYP21A are common, with estimated frequencies as high as 1 in 7 individuals in New York, United States. The estimated prevalence is 1 case per 60 individuals in the general population whilst in selected populations (e.g. the Yupik in Alaska) the prevalence is as high as 1 case in 400 people. Internationally, CAH caused by 21-hydroxylase is found in all populations and occurs among people of all races [3, 4].
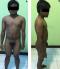
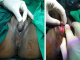
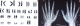
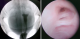
The 15-year-old female patient was born with genital ambiguity. She was brought up as a boy. She is short (146cm) with pubic hair, a vagina, and bilateral unpalpable testes. Karyotype test result was 46,XX, and laboratory examination showed a high value for 17-hydroxyprogesterone (115.9ng/mL). A uterus with endometrial lining was found on USG examination. MRI examination showed a uterus and phallus, and increased bilateral adrenal glands consistent with CAH. Bone age study was consistent with an 18-year-old male. Two orifices were found on endourology examination, an external urethral orifice and a vagina with intact bladder structure. The psychiatric examination concluded that she had an internal conflict due to all examinations being directed to the female gender. However, she still had a tendency to choose female gender (Figure 1, Figure 2).
Accurate diagnosis of a patient with ambiguous genitalia is a challenging process. Based on the diagnosis, a decision will be made for gender assignment, which will have a great impact not only on the patient but also on the patient's family [5]. Management of an infant with a disorder of sex development should include the following: (1) gender assignment must be avoided prior to expert review; (2) evaluation and management should be performed by a multidisciplinary team; (3) gender assignment should be performed as promptly as possible and usually, but not always, in accordance with karyotype; (4) communication with parents, and the patient if possible, must be open, and they should be encouraged to contribute to decision-making [2]. In this case, the parents noticed ambiguous genitalia right after the birth but they only had her examined by a health care provider when she was 12 years old due to cost limitations. The karyotype test result was 46,XX. This means that she does not have a chromosome disorder, but the problem is that overvirilization of a genetic female can be caused by a gonadal or hormone disorder (Figure 3, Figure 4). The majority of virilized 46,XX infants will prove to have CAH. CAH is also the most common disorder of sex development.2 CAH is a group of autosomal recessive disorders that are characterized by a defect in one of five enzymes involved in the synthesis of cortisol, aldosterone or both.1 The most commonly recognized syndromes result from a deficiency of one of the terminal two enzymes of glucocorticoid synthesis (21-hydroxylase or 11-hydroxylase). 6 Deficiency of 21-hydroxylase, resulting from mutations or deletions of CYP21A, is the most common form of CAH, accounting for more than 90% of cases.1,6 The metabolites 17-hydroxyprogesterone (17-OHP) and 17-hydroxypregnenolone, which build up in 21-hydroxylase deficiency, are metabolized to androgens, resulting in virilization of the female external genitalia [5].
Clinically, patients are divided into three categories: classic (0% enzymatic activity, in about 75% of cases), simple virilizing (about 1% enzymatic activity) and non-classic (20-50% enzymatic activity) [5-7]. Each of these disorders is characterized by the activity level of the gene. Patients with the classic disease have both virilization and salt wasting, those with simple virilizing have masculinization without salt wasting and non-classic patients present after puberty with virilization [5] (Figure 5). Simple virilizing 21-hydroxylase deficiency is usually diagnosed in female patients shortly after birth owing to genital ambiguity (CYP21 mutation). Mild forms of 21-hydroxylase deficiency in females are identified later in childhood due to precocious pubic hair, partial or complete fusion of labioscrotal folds, and phallus enlargement to clitoromegaly, and are often accompanied by accelerated growth and skeletal maturation due to excess and postnatal exposure to adrenal androgen, either not treated or inadequately treated. Long-term exposure to high levels of sex hormones leads to premature epiphyseal fusion. Pubic and axillary hair may develop early. Clitoral growth may continue in girls. Long-term exposure to androgens may also activate the hypothalamic-pituitary-gonadal axis, causing centrally mediated precocious puberty [3]. Diagnosis at birth is done by 17-OHP levels on day 3 after birth. In children and adults, diagnosis is based on hormonal levels of 17-OHP, testosterone, DHEAS, androstenedione, cortisol, and plasma renin activity [1]. If 17-OHP levels are > 300mmol/L, classic CAH is likely. A level of 6-300mmol/L indicates non-classic CAH and a patient with a level < 6 mmol/L is likely to be unaffected [8]. Karyotyping is also an important test for diagnosing CAH; it will normally show a female result (46,XX) [9]. The aims of treatment for CAH are to allow normal salt and water balance, avoidance of adrenal crisis, normal blood glucose level, particularly during the newborn period and at times of physical stress, and also to allow normal growth and sexual development [10]. It can be achieved by replacing the deficient hormone. Proper treatment with glucocorticoids prevents adrenal crisis and virilization, allowing normal growth and development [1].
Evidence derived from observational studies suggests that patients with CAH often reach a reduced final height compared to their parentally determined target height. In general, patients have a final height 1 to 2 standard deviations below their estimated genetic potential. Chronic hyperandrogenemia during childhood results in rapid somatic growth with early epiphyseal fusion, ultimately compromising adult stature. Higher doses of glucocorticoids in children with CAH may result in decreased linear growth, especially in early infancy and puberty, when growth velocity is at its peak [11]. Females with ambiguous genitalia require surgical evaluation and, if needed, a plan for corrective surgery. Surgical reconstruction may not be needed during the newborn period in mildly virilized girls but may be appropriate in severely virilized girls, and it should be a single-stage genital repair [1]. Clitorectomy (removal of the whole clitoris) was used in the past [12].The advantage of this procedure is that complete removal of the corpora cavernosa prevents regrowth later in life if the patient is less compliant with hormonal treatment. It was initially advocated because those virilized females with clitorises left intact had erectile tissue that becomes painfully enlarged upon sexual arousal [3]. The clitoris is recognized as an important sensory organ involved in sexual response. Later, surgeons aimed to preserve as much of the enlarged clitoris as possible and buried the corpora under the skin [12]. Instead, a reduction clitoroplasty is favored as it leaves the patient with intact clitoral sensation, painless sexual arousal, a viable and sensate glans clitoris and appropriate erectile function during sexual arousal [3]. The decision of whether or when to perform surgery is often difficult. Some literature says that if surgery is deferred, it must be performed in adolescence [8]. One study also suggested that phallic reduction and recession should be usually performed when the child is 6 to 12 months old and stable from an endocrine standpoint. The two main advantages of early reconstruction surgery are: (1) much better availability and quality of genital tissue, essential material for refashioning the lower genital tract, during the first 6 months of life; (2) the psychological impact of late surgery is more profound than it is during the neonatal period for the child and parent. Early one-stage repair is recommended and has become the standard of care because female patients are able to undergo a more natural psychological and sexual development when they have a vagina with normal appearance [3]. In this case, the operative procedure will be done at 15 years old because she only recently came to a health care provider.
46,XX CAH is a rare case. Management of the patient is based on substantial examination, and the patient's preference has an important role in deciding the type of treatment. In this case, all examinations (physical, laboratory, karyotype, radiology and endourology) were consistent with a diagnosis of CAH, and the patient was categorized as having simple virilizing CAH due to the absence of classical symptoms. The patient has a tendency to identify as female so the operation (clitoroplasty) will be done and the patient will be adapted to become a girl.
The authors declare no competing interests.
All the authors contributed equally in the data collection and the drafting of the manuscript. All the authors read and agreed to the final manuscript.
All Author thank to 10th Malang Continuing Urology Education and Saiful Anwar Hospital for facilitating this article.
Figure 1: clinical presentation of the patient (short with muscle mass and pubic hair): A) anterior view; B) lateral view
Figure 2: A) phallus enlargement; B) orificium urethra externa were identified
Figure 3: A) chromosome analysis (46,XX); B) bone age study showed accelerated growth and skeletal maturation (patient is a 15-year-old female; this was suitable for an 18-19-year-old male)
Figure 4: MRI examination: A) enlargement of bilateral adrenal glands; B) uterine structure
Figure 5: A) genitogram showed female organ genitalia; B) endourology examination: there are two holes, the first is external urethra orifices, the second is vagina
- Othman M, Alali N, Aljayar L. Congenital adrenal hyperplasia: case report. Webmed Central. 2014; 1-4. Google Scholar
- Mark W, Andrew N. Paediatric Surgery II: Disorder of Sex Development. Netherland, Elsevier Ltd. 2013.
- Duarsa GW. Simple Virilizing Congenital Adrenal Hyperlapsia: Presentation in a Female Child with Genital Ambiguity undergoing Genitoplasty. Bali Med J. 2012; 1(3): 93-97. Google Scholar
- Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010; 95(9): 4133-4160. PubMed | Google Scholar
- Baskin L. Disorder of Sex Development. In: Jack WL, Tom FL, editors. Smith and Tanago General Urology 18th Edition. California. 2012. Lange.
- David AD, Richard NY. Sexual Differentiation: Normal and Abnormal. In: Alan JW, Stefanie JT, Jennifer S, editors. Campbell Walsh Urology, Philadelphia Saunders. 2012; 10th Edition.
- Al-Najar MS, Saleem MM, Alassaf AA, Jubouri SA, Aftan MK. Congenital Adrenal Hyperplasia and Ohvira Syndrome: First Report of Unique Combination in a Child. AACE Clin Case Report. 2015; 1(2): e136-e140. Google Scholar
- Phyllis WS, Ricardo A, Laurence SB, Lucia G, Terry WH, Deborah PM, Heino FL, Walter LM, Victor MM, Sharon EO, Martin R, Perrin CW. Congenital Adrenal Hyperplasia due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism. November 2010; 95(11): 5137. Google Scholar
- Puri N, Talwar A. A Case Report of Classic Adrenal Hyperplasia-A Rarity. JIMSA. 2014; 27(1): 51-52. Google Scholar
- Donaldson, Grant, Richard S, Veli F. Hormone and Me: Congenital Adrenal Hyperplasia. Australian Pediatric Endocrine Group, Australia. 2011.
- Leslie JA, Cain MP, Rink RC. Feminizing genital reconstruction in congenital adrenal hyperplasia. Indian journal of urology: J Urol Soci India. 2009; 25(1): 17. PubMed | Google Scholar
- Louise CS, Sarah MC, Adolescent Urogynecology. In: Steffanie JT, Colleen M, editors. Pediatric Urology, Philadelphia Saunders. 2010; Second Edition.