Descriptive study of measles vaccination second dose reporting and barriers to improving coverage in six districts in Malawi
Geoffrey Chirwa, Karen Annette Wilkins, David John Mercer
Corresponding author: Karen Annette Wilkins, Orlando St SW, Atlanta, Georgia 30311, USA 
Received: 11 May 2019 - Accepted: 15 Sep 2019 - Published: 03 Jan 2020
Domain: Epidemiology,Global health,Measles elimination
Keywords: Measles vaccination, measles elimination, measles second dose, data quality audit, data quality assessment, vaccination coverage
This article is published as part of the supplement Innovations in measles and rubella elimination, commissioned by editor@panafrican-med-journal.com.
©Geoffrey Chirwa et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Geoffrey Chirwa et al. Descriptive study of measles vaccination second dose reporting and barriers to improving coverage in six districts in Malawi. Pan African Medical Journal. 2020;35(1):5. [doi: 10.11604/pamj.supp.2020.35.1.19096]
Available online at: https://www.panafrican-med-journal.com//content/series/35/1/5/full
Supplement 
Descriptive study of measles vaccination second dose reporting and barriers to improving coverage in six districts in Malawi
Descriptive study of measles vaccination second dose reporting and barriers to improving coverage in six districts in Malawi
Geoffrey Chirwa1, Karen Annette Wilkins2,&, David John Mercer2
1Ministry of Health, Malawi, 2Independent Consultant
&Corresponding author
Karen Annette Wilkins, Orlando St SW, Atlanta, Georgia 30311, USA
Introduction: Malawiís National Immunization Program introduced a second routine dose of measles containing vaccine (MCV2) in 2015 but found coverage lagging. We assessed data quality and gaps in service delivery.
Methods: investigators used a modified data quality audit in 6 low performing districts accompanied by questionnaires for health facilities (HF) and households with children with >1 vaccination.
Results: MCV2 doses administered according to source were: 733 in registers, 2364 in reports, 1655 in district reports, 2761 in the electronic database. There was 77% agreement regarding status for MCV2 between the register and the home-based record (HBR). Drop-out differences were found between HF according to the practice of waiting for a minimum number of children to open an MCV vial, canceling sessions due to stock-out and requesting payment for a home-based record. Eighty one percent (81%) of children whose caregivers knew 2 doses were needed had received MCV2 vs fifty eight (58%) of children whose caregivers didnít know. Sixty two (62%) of children who were charged for HBR received MCV2 vs 78% reporting no charge.
Conclusion: the drop-out between the first and second doses of MCV was high and inconsistent with elimination goals. The quality of administrative data in these 6 districts was found to be poor. This investigation found that session cancelation, charging for HBR and lack of caregiver knowledge affected completion of the vaccination series. The authors recommend program improvements in these areas to increase uptake of MCV2 and improved reporting practices at all levels of the system.
Measles is a highly contagious disease that prior to widespread vaccination killed an estimate of 2.6 million globally every year [1]. With the introduction of an effective measles vaccine and routine coverage levels over 80%, elimination is considered feasible and a strategic plan to achieve that goal has been developed by the Measles and Rubella Initiative (MRI). In the 2012-2020 plan, MRI calls for countries to ďachieve and maintain high levels of population immunity by providing high vaccination coverage with two doses of measles- and rubella-containing vaccinesĒ and sets a target of 95% coverage with a first and second dose of measles-containing vaccine (MCV1 and MCV2) in each district and every country. In 2011, countries in the African Region of WHO adopted the goal to eliminate measles by 2020 [2]. Vaccination against measles started as a routine program in Malawi in 1980 with one dose given at 9 months of age. Coverage as a percent of children under one year of age vaccinated steadily increased reaching 89% in 2017 [3]. Malawi also conducted 7 national Supplementary measles immunization activities (SIAs) from 2000 to 2017 targeting age ranges defined by the epidemiology of the disease at the time. As a result of routine vaccination and SIAs, the reported number of cases of measles steeply declined from 1989 through 2009 [3]. A large measles outbreak in 2010 as well as a WHO recommendation [4] led the Ministry of Health, National Immunization Program to adopt a second routine dose of measles containing vaccine (MCV2) into the schedule at 15 months of age [5]. The program introduced MCV2 progressively in all districts from July through December 2015. An MCV2 post introduction evaluation (PIE) conducted in October 2016 found that MCV2 coverage for the period January - June 2016 was 57% [6], lagging behind MCV1 coverage and insufficient to reach measles elimination goals [2]. This is consistent with coverage levels of several other countries in the African region as documented by Masresha et al. [7]. However, the PIE was unable to determine if the low coverage was due to poor reporting or to service delivery challenges, leading the Ministry of Health to conduct an additional investigation in February 2017. The objectives of this descriptive investigation were to: a) determine if records of MCV1 and MCV2 doses administered in health facility immunization registers agree with the numbers reported to national level; b) determine if record of MCV2 in the health facility immunization register agrees with the home-based record; c) identify non-data gaps in service delivery that might contribute to non-completion of vaccination among children who began the series.
Six of the countryís 29 districts were chosen based on low MCV2 coverage for the period January - June 2016 as reported through the administrative reporting system plus additional districts chosen to provide an urban/rural mix. Within each district, a simple random sample of 3 health facilities (HF) was chosen. Using a modified data quality audit methodology [8], the number of doses of tracer vaccinations (MCV1 and pentavalent (diphtheria, pertussis, tetanus, hemophilus influenzea type B, hepatitis B) vaccine administered to children less than 12 months and more than 12 months of age, and MCV2) was collected from immunization registers, tally sheets and monthly reports at the facility and districts level; and from electronic databases for those facilities at the district and national levels for the months of February, October and December 2016 using standardized tools. A standardized questionnaire covering vaccination practice was administered to Health Surveillance Aids (HSA) in each HF.
†
To identify children who had begun their primary vaccination series, a simple random sample of 2 communities per HF was chosen by the interviewers using the facility immunization register. All children: a) from those communities; b) born between December 1, 2014 and August 30, 2015 and c) who received at least one vaccination between January 1 and October 31, 2015 were listed and 6 systematically selected for household visits. If there were less than 6 children from a chosen community, a neighboring community was selected based on local knowledge of geography. Households were visited and care givers of children identified from the register were interviewed using a standardized questionnaire and the information in the home-based vaccination record was copied. For purposes of comparing HF, drop-out between MCV1 and MCV2 was calculated from household questionnaires and HF divided into higher and lower drop-out in relation to the mean rate. One HF for which household questionnaires were not completed was excluded from this analysis. Data were collected using tablets preloaded with questionnaires in ODK and analyzed using MSExcel and EpiInfo.
Information was successfully collected at the national level, in all 6 districts and 18 health facilities. After deduplication, there were 189 child records from the immunization registers of 17/18 health facilities and from 38 communities. The immunization register was not useable for child identification in the 18th HF. The health facilities interviewed were 2 hospitals, 12 health centers, 2 dispensaries, 1 clinic and 1 health post. Of the 189 children sought from the register, there was 1 death, 1 refusal and 24 who were not located. Of the 163 interviews, four children no longer lived in the location in the register resulting in 159 completed household questionnaires. The age of the children included in the sample provided a range from 17 through 26 months of age at the time of the survey. There were 6 to 14 household questionnaires per health facility.
†
Data quality†
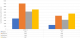
Immunization registers were available in all health facilities visited. Tally sheets were present and being used in 1/18 HF. Completed monthly reports were found for all 3 months in 17 HF, in the 18th HF 2 completed forms were located. Monthly report forms with printed space for MCV2 were available in 17/18 HF. 7/18 HF reported stockouts of reporting forms for the monthly report and or vaccination cards in 2016 ranging from 1 to 6 months with an average of 3 months. The number of doses recorded administered were collected from HF. As can be seen in Table 1, a total of 1872 doses of MCV1 administered were found in immunization registers and 4550 found in monthly reports. For MCV2, a total of 733 doses administered were found in immunization registers and 2364 found in monthly reports. A total of 4550 doses MCV1 administered were found in health facility monthly reports located at the district level and 3109 found in monthly reports prepared by the district with 10 exactly agreeing. A total of 2362 doses MCV2 administered were found in HF monthly reports located at the district and 1655 found in monthly reports prepared by the district level with 8 exact matches. A total of 3109 doses MCV1 were found in the monthly reports for the health facilities prepared by the district and 3487 in the electronic database at the national level for the 18 health facilities. For MCV2, a total of 1655 doses administered were reported per monthly reports and 2761 per the electronic database. The differences between total numbers of doses administered by source is illustrated in Figure 1. There was 76% and 77% agreement regarding vaccination status for MCV1 and MCV2 respectively between the register and the home-based record (HBR) without taking dates into consideration (Table 2). More children were vaccinated per HBR (128 vs. 97 MCV1; 78 vs. 70 MCV2) than per the immunization register.†
Factors affecting MCV2 coverage†
The 17 HF with household questionnaires were compared for factors that might influence drop-out (Table 3). Health facilities with less than average drop-out rates planned an average of 8.7 static and 4.6 outreach under-2 well child clinics per month and offered MCV at all sessions. Seventy eight (78%) reported that they had no minimum number of children required to be present to open a vial of measles vaccine. Twenty-two percent reported that they had canceled at least one vaccination session due to stock-out of vaccine. This question was not specific to measles vaccine. Eleven percent stated that they prioritized who would receive measles vaccine if stocks were low. Eleven percent reported that they requested payment for the home-based record, none requested payment for services. The 8 health facilities with greater than average drop-out planned an average of 11.6 static and 3.1 outreach under-2 well child clinics per month, MCV was offered at 87.5% of static and 100% of outreach sessions. All reported that they had no minimum number of children to open a vial of measles vaccine. Sixty-two percent had canceled at least one vaccination session due to stock-out of vaccine. This question was not specific to measles vaccine. Zero percent stated that they prioritized who would receive measles vaccine if stocks were low. Sixty-two percent reported that they requested payment for the home-based record, none requested payment for services.†
Among the children included in the household survey, 9 MCV1 vaccinations were misrepresented as MCV2 (in the absence of a first dose, older than one year at the time of vaccination). These were corrected to MCV1. 146/159 children (91%) had a home-based vaccination record present at the time of the survey, 143 of these were the government-issued passport and 3 a vaccination card or other record. Vaccination status was estimated from the home-based record and parental recall. 150/159 (94%) of children had received MCV1 and 106/159 (67%) MCV2 based on the home-based record or care-giver recall - a drop-out rate of 29% (Table 4). Sixty-two percent of respondents thought that the child had received all of his/her vaccinations, 71% of whom had received MCV2. Ninety-eight percent of respondents had heard of measles vaccination. Forty-three percent knew that a child needed 2 doses of MCV. Of those that said a child needed 2 doses, 81% had received MCV2. Of the 53% (85/159) who thought the child needed one dose or said that they did not know 58% had received MVC2. New HBR were printed for the introduction of MCV2 but not all children were in possession of the new cards. Sixty-eight percent of those that had the new cards with MCV2 pre-printed had received that dose. Sixty-seven percent of those with the HBR without MCV2 had received that dose. Of those who reported that they paid for the HBR, 62% had received MCV2. Seventy-eight percent who reported they had not paid for HBR had received MCV2 (Table 4). Eighty-nine percent of caregivers said that the health care worker (HCW) was the most trusted source of information regarding vaccination and 79% that they knew when to return for a vaccination because the HCW had told them during the previous clinic visit.
We confirmed that drop-out between the first and second doses of MCV is a problem in the 6 districts included the study. Drop-out ranged from 21% to 61% depending on the data source used, all higher than levels needed to achieve the measles elimination goal endorsed by the national, regional and global levels. This study determined that records of MCV1 and MCV2 doses administered were inconsistent from HBR to HF registers to HF monthly reports, through the district level reports to the national database. This lack of consistency undermines the confidence of program managers at all levels. The figures available at district and national levels were 1.7 - 2.4 times higher than those found in immunization registries, masking the probable low coverage in the HF and the high drop-out rates limiting the use of the data for planning or evaluation. The immunization register at the HF is a key tool in identifying children who have missed doses for follow-up and, in the absence of tally sheets, is the only source of information for the monthly reports [9]. In one of these HF, the immunization register was not name-based, making defaulter tracking impossible. In the other 17, immunization registers missed 25% of the MCV1 doses and 26% of the MCV2 doses found in HBR.
†
This investigation was not designed to provide a coverage estimate for the districts included. Based on the design, which was intended to determine the quality of reported information and identify potential factors contributing to high drop-out between MCV1 and MCV2, only children who had initiated the vaccination series and were identifiable from the immunization registry were included. A variable number of households were interviewed by HF further limiting interpretation of the responses. Therefore, results of the household component of the investigation are not representative of the HF nor the districts but have provided hypotheses for high drop-out which can be further tested.†
While not representative, this investigation found that drop-out was higher than average in HF that more frequently reported cancellation of at least one vaccination session due to stock-out and who reported they charged for HBR. The household questionnaires also identified care giver reports of paying for the HBR as potentially resulting in less MCV2 coverage. The HBR is an essential tool for reminding care givers of vaccination history and facilitating screening for vaccinations due during immunization sessions or curative care [9, 10]. The Malawi national policy is consistent with WHO recommendations and common practice [11]. However, this investigation demonstrates that the policy is not universally followed and may have a negative impact on completion of the vaccination series. Efforts must be undertaken to assure the respect of national policy and provision of HBR free of charge.†
Caregiver knowledge of the need for a second routine dose of measles vaccine was another factor identified as potentially affecting MCV2 coverage with 81% of those who knew receiving MCV2 vs 58% of those who thought one dose was needed or replied that they did not know. While interpretation of this observation is further limited by directionality (mothers of children who received 2 doses are more likely to know about the need for 2 doses), HCW are the most trusted source of information regarding immunization in general and the next appointment in particular. HCW must increase communication about the need for a second dose of MCV.
In summary, we found that the drop-out rate between the first and second doses of MCV was high and inconsistent with measles elimination goals. We also found that the quality of administrative data in these 6 districts was poor and greater attention needs to be paid at all levels to improve the collection and use of data. Additionally, this investigation found that session cancelation, charging for HBR and lack of caregiver knowledge are potential factors affecting completion of the vaccination series. These hypotheses may be tested through additional studies. Authors recommend undertaking program improvements that focus on these areas to increase uptake of MCV2 and improve reporting practices at all levels of the system are logical and recommended. Following this investigation, the immunization program began implementation of missed opportunity for vaccination [12] and second year of life [13] strategies in 3 districts.
What is known about this topic
- Coverage with a second routine dose of measles containing vaccine lags behind coverage with the first dose in many countries, including Malawi;
- Health worker behavior does not always follow national policies.
What this study adds
- Addressing the challenge of low coverage and high drop-out between measles doses is complicated by poor data quality;
- Despite national policy on providing home-based-records free of charge, some HF continue to charge which seems to have an effect on completing MCV2;
- Frequent cancelation of sessions due to stock-out may have an effect on drop-out rates.
The authors declare no competing interests.
Geoffrey Chirwa, acting director of the National Immunization Program at the time of the PIE and investigation, conceptualized the investigation and oversaw all logistical operations, while taking on the function of national supervisor. Mr. Chirwa contributed significantly to commenting on the drafts of the paper. Karen Wilkins was co-principal investigator on the investigation, secured financing and was principal writer of the manuscript. David Mercer provided key contributions to the manuscript drafts and oriented the data analysis. All authors have read and agreed to the final manuscript.
The authors would like to acknowledge the contributions of Dr. Kwame Chiwaya who was an active participate in all aspects of this investigation but whom we lost too soon. Ms. Sara Andrist provided assistance with the collection of data quality information, managing the tablets and supervising teams. Funding for this investigation was kindly provided by Global Alliance for Vaccines and Immunizations (GAVI) through the Centers for Disease Control and Prevention and the World Health Organization.
Table 1: doses of Measles containing vaccine (MCV) administered by Health Facility and by information source, February, October and December 2016
Table 2: vaccination status by source of information: home based record and immunization register
Table 3: selected reported service delivery behavior for health facilities with less than and greater than average drop-out rate
Table 4: vaccination coverage by source and selected care-giver responses by vaccination status, MCV2 (card + history)
Figure 1: total doses
MCV1 < 1 and MCV2 administered by source, 18 health facilities
- World Health Organization. Measles. 29 November 2018. Accessed on 19 Apr 2019.
- World Health Organization. Global measles and rubella strategic plan 2012-2020. Accessed on 19 Apr 2019.
- World Health Organization. WHO vaccine-preventable diseases: monitoring system. 2019 global summary. Accessed on 19 Apr 2019.
- World Health Organization. Weekly Epidemiological Record (WER). Weekly Epidemiological Record. Accessed on 19 Apr 2019.
- Government of Malawi, Ministry of Health, Expanded Programme on Immunization, EPI Comprehensive Multi-year Plan. 2016-2020. September 2015.
- Malawi EPI, World Health Organization, UNICEF, Measles Second Dose Post-Introduction Evaluation Report. October 2016.
- Masresha BG, Luce R, Okeibunor J, Shibeshi ME, Kamadjeu R, Fall A. Introduction of the second dose of measles containing vaccine in the childhood vaccination programs within the WHO Africa Region - lessons learnt. J Immunol Sci. 2018;S(17):113-121. PubMed | Google Scholar
- World Health Organization. The immunization data quality audit (DQA) procedure. November 2003. Accessed on 19 Apr 2019.
- World Health Organization. Handbook on the use, collection, and improvement of immunization data. Draft for comments. June 2018.
- Brown D, Gacic-Dobo M. Occurrence of home-based record stock-outs - a quiet problem for national immunization prrogrammes continues. Vaccine. 2018 Feb 1; 36(6):773-778. PubMed | Google Scholar
- Young S, Gacic-Dobo M, Brown D. Results from a survey of national immunization programmes on home-based vaccination record practices in 2013. Int Health. 2015 Jul; 7(4):247-255. PubMed | Google Scholar
- World Health Organization. MOV, Planning guide to reduce missed opportunities for vaccination. 2017. Accessed on 10 May 2019.
- World Health Organization and UNICEF. Establishing and strengthening immunization in the second year of life, practices for vaccination beyond infancy. 2018. Accessed on 10 May 2019.
Search
This article authors
On Pubmed
On Google Scholar
Citation [Download]
Navigate this article
Similar articles in
Key words
Tables and figures
This supplement
- Control of measles in a juvenile custodial setting in the wake of the recent US outbreak (Accessed 1331 times)
- The impact of a prolonged ebola outbreak on measles elimination activities in Guinea, Liberia and Sierra Leone, 2014-2015 (Accessed 1280 times)
- Measles and rubella microarray array patches to increase vaccination coverage and achieve measles and rubella elimination in Africa (Accessed 1029 times)
- Temporal trend of measles cases and impact of vaccination on mortality in Jigawa State, Nigeria, 2013-2017: a secondary data analysis (Accessed 1003 times)
- The use of WhatsApp group messaging in the coordination of measles supplemental immunization activity in Cross Rivers State, Nigeria, 2018 (Accessed 997 times)
- Global trends in measles publications (Accessed 875 times)
- Control of measles in a juvenile custodial setting in the wake of the recent US outbreak (Downloaded 259 times)
- The impact of a prolonged ebola outbreak on measles elimination activities in Guinea, Liberia and Sierra Leone, 2014-2015 (Downloaded 245 times)
- Global trends in measles publications (Downloaded 192 times)
- Temporal trend of measles cases and impact of vaccination on mortality in Jigawa State, Nigeria, 2013-2017: a secondary data analysis (Downloaded 189 times)
- Measles and rubella microarray array patches to increase vaccination coverage and achieve measles and rubella elimination in Africa (Downloaded 187 times)