Blood donor safety, prevalence and associated factors for cytomegalovirus infection among blood donors in Minna-Nigeria, 2014
Musa Kalamullah Bawa, Aisha Mamman, Adebola Olayinka, Saheed Gidado, Ndadilnasiya Endie Waziri, Muhammad Shakir Balogun, Kabir Ibrahim Getso, Mahmood Muazu Dalhat, Peter Nsubuga, Nuruddeen Aliyu, Hussaini Bala, Hauwa Muhammad, Suleiman Haladu, Usman Lawan Shehu, Patrick Mboya Nguku
Corresponding author: Musa Kalamullah Bawa, Nigeria Field Epidemiology and Laboratory Training Program (NFELTP), Abuja, Nigeria 
Received: 09 Jul 2017 - Accepted: 04 Dec 2017 - Published: 22 Jan 2019
Domain: Epidemiology,Polio eradication,Public health
Keywords: Cytomegalovirus, blood donors, Minna, Northern Nigeria
This article is published as part of the supplement Sharing Experiences from the Field : Updates from the Nigeria Field Epidemiology and Laboratory Training Program, commissioned by African Field Epidemiology Network (AFENET) Nigeria.
©Musa Kalamullah Bawa et al. Pan African Medical Journal (ISSN: 1937-8688). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Musa Kalamullah Bawa et al. Blood donor safety, prevalence and associated factors for cytomegalovirus infection among blood donors in Minna-Nigeria, 2014. Pan African Medical Journal. 2019;32(1):6. [doi: 10.11604/pamj.supp.2019.32.1.13297]
Available online at: https://www.panafrican-med-journal.com//content/series/32/1/6/full
Research 
Blood donor safety, prevalence and associated factors for cytomegalovirus infection among blood donors in Minna-Nigeria, 2014
Blood donor safety, prevalence and associated factors for cytomegalovirus infection among blood donors in Minna-Nigeria, 2014
Musa Kalamullah Bawa1,&, Aisha Mamman2, Adebola Olayinka1,2, Saheed Gidado1, Ndadilnasiya Endie Waziri1, Muhammad Shakir Balogun1, Kabir Ibrahim Getso1,3, Mahmood Muazu Dalhat1, Peter Nsubuga4, Nuruddeen Aliyu1, Hussaini Bala1, Hauwa Muhammad1, Suleiman Haladu1, Usman Lawan Shehu1, Patrick Mboya Nguku1
1Nigeria Field Epidemiology and Laboratory Training Program, Abuja-Nigeria, 2Ahmadu Bello University, Zaria, Nigeria, 3Ministry of Health, Kano, Nigeria, 4Global Public Health Solutions, Atlanta GA, USA
&Corresponding author
Musa Kalamullah Bawa, Nigeria Field Epidemiology and Laboratory Training Program (NFELTP), Abuja, Nigeria
Introduction: human cytomegalovirus (CMV) has remained a cause of morbidity and mortality in pregnancy and immunocompromised patients. CMV is transmissible through blood transfusion. We conducted a descriptive, cross-sectional study to assess blood donor safety and to determine the prevalence and associated factors for CMV infection among blood donors in Minna, Nigeria.
Methods: all consenting blood donors were screened for CMV antibodies (IgM and IgG) using ELISA kit and haematological indices using a haematological analyzer. We administered structured questionnaires to obtain socio-demographic and socio-economic data. Data were subjected to univariate, bivariate and multivariate statistical analyses using Epi Info version 3.5.4. Significant associations were presumed if p < 0.05.
Results: a total of 345 participantswere recruited, the majority were males 336 (97.4%). Monthly earnings of majority of the blood donors, 136 (40.6%) ranged from ₦18,000 to ₦35,000. The prevalence of CMV infection was 96.2%. The prevalence of anti-CMV IgG antibodies was 96.2% and that of IgM was 2.6%. Most of the study participants, 274 (79.4%) were family replacement donors. The majority of the blood donors 195 (56.5%) were anaemic (PCV < 36, Hb < 12g/dl). Those with positive CMV were more likely to be of high-income level (OR = 0.32, P = 0.04).
Conclusion: the seroprevalence of CMV was high with a significant proportion of donors capable of transmitting CMV infection to blood recipients. The majority of the blood donors were anaemic. High income level is associated with CMV infection. Quality of screening for anemia be improved.
Blood transfusion is usually a lifesaving therapeutic intervention. However, many preventable errors may make this a hazardous procedure [1]. The World Health Organization (WHO) recommends that blood donation should in all cases be voluntary [2]. However, in Nigeria, voluntary donors are relatively scarce. Hence, family replacement and commercial donors have become alternative sources of blood [3]. Healthy persons who are between the ages of 18 and 65 years with haemoglobin (Hb) levels of not less than 13.5 g/dl in males or 12.5 g/dl in females are acceptable as donors if they test negative for transfusion-transmissible infections (TTIs). These TTIs include hepatitis B virus (HBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV), malaria, syphilis and chagas disease. However, females are only accepted as donors if they are not pregnant or breastfeeding [4]. Human cytomegalovirus (CMV), otherwise called human herpes virus type 5, over the years, has come to assume an important public health problem. As it is a significant cause of morbidity and mortality in pregnancy and among immunocompromised patients like recipients of organ transplants, HIV-infected persons, cancer patients on therapy and neonates [5].
Cytomegalovirus is transmissible through blood transfusion, among other parenteral routes [6]. However, donor screening for CMV is not routinely undertaken in Nigeria. A study by Chakravarti shows that most adults across the globe are seropositive for CMV [6]. The African region of the WHO, fraught with high disease burden and high prevalence of TTIs is faced with unique challenges to blood safety [7]. CMV is part of this challenge. A study in Ghana reported a CMV prevalence of 93.2% [7]. A previous study on the pre-donation screening of intending blood donors for antibodies to infectious agents at Obafemi Awolowo University Teaching Hospital Complex, Ile-Ife, Osun State, Nigeria indicated that about 12% donors were deferred due to TTIs and 5% due to anaemia [8]. The prevalence of transmissible transfusion viruses is still very high in Nigeria when compared with other developing countries with very similar challenges [9]. This challenge needs to be addressed to ensure blood and blood product safety. CMV prevalence rates in some parts of Nigeria were from 92% in Jos to 95% in Lagos both in 2009 [10].
The symptoms of CMV infection vary depending on the age and health of the person who is infected, and how the infection occurred and include hearing, vision, neurological and developmental problems. Other symptoms and morbidities of CMV include premature delivery, jaundice, spleen, microcephaly (small head), seizures, rash and feeding difficulties [8]. Premature and ill full-term infants who are infected soon after birth are also at risk for neurological and developmental problems over time [8, 10]. Transfusion transmitted-cytomegalovirus (TT-CMV) is a significant cause of morbidity and mortality in the immunocompromised host. The risk of TT-CMV from seropositive donors is reported to be 0.4 to 12% [11]. The blood transfusion screening algorithm in Minna, Niger State includes HIV, HBV, HBC and syphilis. Malaria is endemic and anaemia is a common diagnosis in the area [12]. We conducted a study to determine the prevalence of and associated risk factors for CMV infection in blood donors in Minna, Northern Nigeria.
Study area: the study was conducted in Minna, the capital city of Niger State, Nigeria. The city is located in the North Central region of Nigeria. Minna City is made up of two local government areas (LGAs), each having one secondary health facility, General Hospital (GH), Minna and Ibrahim Babangida Specialist Hospital (IBBSH), Minna located in Minna South and Minna East LGAs respectively. The average annual blood donor rates were 3,865 blood donors for GH, Minna and 756 for IBBSH, Minna. The population of Minna is 304, 113 projected from 2007 census.
Study design: we conducted a hospital-based cross-sectional study with both descriptive and analytical components. The descriptive component described the occurrence of CMV among the blood donors regarding person, place and time, while the analytical component identified the factors associated with CMV infection.
Study population: the study population was all potential blood donors that presented at the identified blood donation centers, GH Minna and IBBSH, Minna, Niger State.
Eligibility criteria: we included apparently healthy looking persons who were between the ages of 18 and 65 years with haemoglobin (Hb) levels of not less than 13.5 g/dl in males or 12.5 g/dl in females and those already screened (laboratory screening) for transmission transmissible infections and found eligible to donate. The TTIs considered excluded CMV as donor screening for CMV is not routinely undertaken in Nigeria. Apparently healthy looking persons were considered to be people apparently looking well fed devoid of physically observable signs of sickness or disease.
Exclusion criteria: all those that tested positive for HIV, HBV and HCV were excluded.
Sample size determination: a total of 345 blood donors were recruited into the study. The sample size was obtained using the following formula:
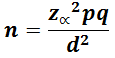
Where: n= required sample size, our Z (z = (1-a/2)) value was 1.96. This represents the value of the standard distribution corresponding to a significance level of α (1.96 for a 2-sided test at the 0.05 level). We used a prevalence (p) of 92% (0.92) obtained from CMV prevalence study among prospective blood donors at a tertiary health facility (Jos University Teaching Hospital) in Jos, Nigeria [5]. Our q (1-p) was 0.08 and our absolute precision (d) was 3% (0.03).
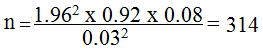
Using 10% non-response obtained as 31, we obtained the sample size (n) 345.
Sampling technique
We employed systematic random sampling technique. We proportionately allocated the participants to the health facilities, GH, Minna and IBBSH, Minna using the sampling frames for the health facilities. The blood donors that fitted into the inclusion criteria of the study and consented to the study were systematically selected from the two health facilities after obtaining an interval for selection for each, using n/N = K. The interval was 5 and the first participant was selected randomly by balloting between 1 and 5, and then after that every Kth blood donor recruited until the required sample was obtained.
Study instruments
We used laboratory forms for capturing laboratory data, and interviewer-administered questionnaires made up of demography and risk factor sections were used for capturing data on socio-demographic, socio-economic characteristics and practices of the participants.
Data collection methods
Data were collected by trained research assistants and laboratory scientists. The data collection was from November 2013 to January 2014. 5ml of whole blood was collected using EDTA and serum using plain vacutainers from each subject. The serum was used for CMV ELISA screening for CMV antibodies, i.e. IgG and IgM using ELISA kit (DIALAB® Austria), while the whole blood was analyzed for haematological indices using a haematological analyzer (Abacus junior haematology analyzer 2.75, manufactured in 1995 by Diatron® U.S.A). We used interviewer-administered questionnaires to obtain information from participants regarding their socio-demographic, socio-economic characteristics and practices [8].
Screening for CMV
Screening for CMV antibodies was done using ELISA kit (DIALAB® Austria). Serum was preserved under -20°C and analyzed using ELISA. The analysis was done using automated microplate reader (Emaxprecision microplate reader), E11865 model (Molecular Devices® USA). ELISA procedure is shown in detail in an attached Annex 1. No molecular testing was done.
Analysis for haematological indices
Blood samples for haematological analyses were analyzed daily within 12 hours of collection using a haematological analyzer (Abacus junior haematology analyzer 2.75). The procedure included: 1) Using the EDTA blood container, whole blood was inserted into the analyzer; 2) The analyzer aspirated 2 micro liters of the blood into the probe and autoanalysed; 3) The result appeared on the screen and copied.
Data management
The independent variables were the socio-demographic and socio-economic characteristics of the respondents, cultural practices, while the dependent variable was the CMV serological status of the blood donors regarding IgM and IgG, which are either positive or negative. The socio-demographic and socio-economic characteristics included age, sex, occupation, educational status, ethnic group, marital status, past history of transfusion, type of donor and monthly income level. The cultural practices included tribal marks, received blood transfusion, received surgical procedure in HF, local circumcision, local belubelu (uvulectomy), dental procedure inside HF, dental procedure outside HF, worked in contact with blood and sex partner not spouse.
We reviewed all the completed questionnaires before electronic entry. Data obtained were analyzed using Epi Info version 3.5.4 (US Centers for Disease Control and Prevention) and Microsoft Excel 2007. Significant associations were presumed if p < 0.05.
Ethical considerations
Ethical clearance was obtained from the GH, Minna and IBBSH, Minna researchand ethical clearance committees. Respect to participants´ rights was observed including the right to refuse participation with explanation through participant´s information form. We conducted informed consent for all potential participants prior to study through provision of individual consent forms for their consent.
The majority of the blood donors, 139 (40.3%) were aged 20-29 years, 230 (66.7%) were married, 65 (19.5 %) were unemployed, 273 (79.4%) were family blood donors. Most, 146 (42.6%) had post-secondary education, and 136 (40.6%) had a monthly income of between $51 and $100 (Table 1). The prevalence of CMV infection was found to be 96.2%. The prevalence of CMV IgG was found to be 96.2%, and that of CMV IgM was 2.6%. Combined CMV IgG and CMV IgM antibodies were detected in 9 (2.6%) blood donors (Table 2). Analyses of the distribution of CMV IgG seropositivity with age showed blood donors aged 20-29 years had the highest CMV IgG seroprevalence, 40.4% closely followed by those aged 30-39 years with 40.1%. The least CMV IgG seroprevalence, 0.9% was found in the blood donors aged > 60 years (Figure 1).
Bivariate analysis for socio-economic factors for CMV (IgG and IgM) infection showed blood donors with monthly income level < ₦18000 were less likely to be CMV-positive than those with higher income (OR = 0.32 (95% confidence interval: 0.10-0.97)). Other factors, age, sex, marital status, type of marriage, educational level, and occupation showed various levels of association but were not significant (p > 0.05) (Table 3). Table 4 shows the pattern of haemoglobin concentration (Hb) and packed cell volume (PCV) among various strata of blood donors. Of the 345 blood donors, 227 (65.8%) had Hb below the normal range (i.e. Hb < 12g/dl). Family replacement blood donors formed the majority, 185 (81.5%) of the anaemic blood donors. Most blood donors (197, 56.5%) had PCV below the normal range (i.e. PCV less than 36%). None of the blood donors had Hb or PCV above the normal range. Analysis for variations and statistical significance of means of CMV seropositive group and CMV seronegative group on haematological indices showed CMV seropositive group had a low Hb (p = 0.02), PCV (p = 0.03) and mean platelet distribution width (MPDW) (p = 0.04) compared to those of CMV seronegative group (Table 5).
We aimed at assessing the blood donor safety and determining the prevalence and associated factors for CMV infection among blood donors in Minna, Nigeria.We found the seroprevalence of CMV to be 96.2%; this is consistent with findings of a study in Sfax region, Tunisia (97.1%), Jos, Nigeria (92%), and Lagos, Nigeria (96%) [5, 11, 12]. The finding indicates CMV infection is widely spread among the human population but not commonly known as most CMV infections are asymptomatic and therefore commonly go undiagnosed [13]. The high prevalence of anti-CMV IgG antibodies found in this study corroborates findings of other studies in Southern Brazil (96.4%),Ghana (93.2%), Ilorin (96.7%), and Lagos, Nigeria (96%) [8, 12-14]. We found the prevalence of anti-CMV IgM antibodies as 2.6%; this is lower than 5.5% reported at Albania and 3.1% at Benin, Nigeria [10]. Studies with nearly comparable anti-CMV IgM antibody rates include 2.3% in Brazil and 2.5% in Sudan [15, 16]. CMV IgM prevalence represents the proportion of those with reactive or ongoing CMV infection and are capable of infecting others: This reflects the role of CMV as a TTI [14].
Combined CMV IgG and CMV IgM antibodies were detected in 9 (2.6%) participants, a figure that is higher than 1.6% reported by Chaudhari and Bindra [17]. Re-activation infection is associated with a CMV IgM response. Individuals who suffer a re-activation have previously mounted a CMV IgG response. Therefore, anti-CMV IgG prevalence rates are considered to reflect the overall prevalence for epidemiologic purposes; as CMV IgG reflects a chronic infection [5, 6, 14]. CMV IgG seropositivity distribution with age showed CMV seroprevalence deferred with age. We found the blood donors aged 20-29 years had the highest CMV seroprevalence of 40.4% closely followed by those aged 30-39 years, 40.1% while the least was found in those aged > 60 years as 0.9%. Conversely, younger blood donors aged < 20 had CMV seroprevalence of 1.8%. This is very similar with the finding by Alao where the peak age CMV seroprevalence was in blood donors aged 25-29 years age, which represented 30.4% of the study participants. Our finding is also closely similar with another study which had the highest CMV serprevalence in blood donors aged 30-39 as we found the age group 30-39 years ranked the second highest CMV seoprevalence [10]. CMV seroprevalence was lowest in those aged 15-19 years and above 50 years(1.6% each) [5]. However, our finding contrast a finding by Wujcicka and others which indicates that CMV seropositivity is significantly associated with increasing age [18]. Given that CMV typically leads to lifelong seroconversion, it will be expected that CMV seroprevalence will increase with age. The difference in the findings might have to do with the study populations and need to be explored further.While our study population was potential blood donors that presented at the identified blood donation centers aged between 18 to 65 years, the study by Wujcicka and others was in a cohort of pregnant Polish women.
Our study found family replacement donors constituted the majority, 79.4% while commercial donors made up 3.2% and voluntary donors 17.4%. The low proportion of voluntary donors reflects the duo role of ignorance and low national development index [2]. Family replacement blood donation predominates in the absence of a well organized national voluntary blood donation programme. People then rely on family or friends of patients to act as replacement donors. However, research findings indicates that blood from family or replacement donor is found to be unsuitable more often than blood from voluntary non enumerated and therefore presents a potentially greater risk to the safety of the blood supply [19, 20]. The commonest age group in this study was 20-29 years; this is higher than 18-20 years reported by a study in Chennai, India [21]. Our finding showed an overwhelming male predominance (97.4%). It is comparable to 95.4% reported by Akinbami and colleagues [14]. It maybe linked to the belief that women do not donate blood because of menstrual flow, pregnancy and childbearing [22].
We found that most study participants were employed, retirees accounted for very few. It shows blood donation is an activity of persons < 65 years of age [2]. The majority of the blood donors were educated as those without education were 21.9%. The rate of illiteracy observed in our study is higher than 15.4% reported for the North-Central zone in the National Demographic and Health Survey (NDHS) 2008 by National Population Commission, Nigeria (NPC) [23]. People earning of ₦18,000 and above were more likely to be CMV antibody positive. Blood donors with a monthly income level less than ₦18,000 were 68% less likely to be CMV-positive than those with monthly income level equal to or higher than ₦18,000. Our finding is consistent with findings by Revello and Giuseppewhere they found that the risk of primary maternal infection of CMV was about three times higher among the higher-income susceptible women (45%), compared to 15% in the lower-income group [24]. This finding is contrary to the report in California in which persons who earned less than $1,000 had a risk of 43.5% more than individuals with higher income [25]. Our finding also contrasts other findings that showed that the major risk factor for CMV infection is exposure to children [18, 26-28]. Many of the blood donors in this study, 55.9% were found to be anaemic (PCV < 36%). The study participants were considered to have been screened (laboratory screening) for anaemia with PCV cutoffs as not less than 40.5% (13.5 g/dl) in males or 37.5% (12.5 g/dl) in females based on the blood donor eligibility criteria of the country. Yet, such a high level of proportion of anaemic blood donors was found. This goes to show the level of the quality issues associated with screening procedures. The high proportion of anaemia in our study corroborates findings of other studies which include studies in Port Harcourt, Nigeria and south India [29, 30]. This is also identical with findings of Xu and colleagues, Gordon-smith and associates and Taglietti and colleagues [31-33]. This high level of anaemia may be associated with the finding in the study that 81.5% of the anaemic donors were family replacement donors and family replacement donors constituted 79.4% of all the donors (345) in the study.
Comparison of the difference of the means of PCV and Hb between CMV seropositive and CMV seronegative donors showed CMV seropositive donors had a lower PCV and Hb (P < 0.05). CMV seropositivity with its attendant risk predisposes to anaemia [34]. CMV causes infection of bone marrow suppression which is a risk factor for aplastic anaemia [35-37]. The findings of our study cannot be generalized to Nigeria as the study was a health facility-based study. Also, most of the private health facilities were not consistent blood donation centers and therefore were not considered in the study as their inclusion could have constituted a bias. Our findings will still provide the basis for the implementation of donor safety strategies in Minna.
In conclusion, we observed a high seroprevalence of 96.2% of CMV in Minna among blood donors with a significant proportion (2.6%) capable of transmitting CMV infection to blood recipients. But since up to 96.2% of blood donors are seropositive for CMV, it would seem superfluous to screen blood donors for CMV for all transfusions, as few seronegative blood units would be available for transfusion. The majority of the blood donors were anaemic. Prospective blood donors for immunocompromised patients, however, should be screened for CMV. The quality of screening for anemia should be improved.
What is known about this topic
- Transfusion is a lifesaving therapeutic intervention - However, many preventable errors may make this a hazardous procedure;
- Cytomegalovirus is transmissible through blood transfusion, among other parenteral routes, however, donor screening for CMV is not routinely undertaken in Nigeria;
- CMV infection is widely spread among the human population but not commonly known as most CMV infections are asymptomatic and therefore commonly go undiagnosed.
What this study adds
- Combined CMV IgG and CMV IgM antibodies are detected in 2.6% of blood donors;
- Blood donors with monthly income level < ₦18000 are less likely to be CMV-positive than those with higher income;
- More than half of the study participants (blood donors) (65.8%) were anaemic i.e. had Hb below the normal range (i.e. Hb < 12g/dl) and majority were family replacement blood donors.
The authors declare no competing interests.
MB was the principal investigator in this study from proposal, design and protocol development through data analysis to the final manuscript writing. AM contributed in the review of the article. AO contributed in the study design and review of the article. SG, NEW contributed in the design and review of the article. MSB, KIG, MMD, PN, NA and HB supported in the study design, data analysis and contributed in the review of the article. HM, SH, ULS contributed in the draft and review of the article. PMN contributed in the research protocol development, design, draft and review of the article. All authors have read and agreed to the final version of this manuscript.
We wish to acknowledge Professors Kabir Sabitu, Gabrielle Poggensee (Nigerian Field Epidemiology and Laboratory Training Program (NFELTP), professor Elizeus Rutebemberwa (Makerere University) for their mentoring support in this work. I want to acknowledge my NFELTP field supervisor Dr Tijjani Hussein. We also acknowledge the contributions of Alhaji Alfa Dangana and Dr. James Kolo (Niger State Ministry of Health) for their technical support. We acknowledge the cooperation of the staff of haematology department, GH, Minna and antiretroviral therapy (ART) (President Emergency Plan for AIDís Relief - PEPFAR) laboratory, ABUTH, Zaria for enabling us haematological and serological analysis. We also acknowledge the contributions of the research assistants, AbdullahiHabibu, Abdullahi Sanusi of GH, Minna and Yusuf Ahmadu of IBBSH, Minna.
Table 1: sociodemographic characteristics of blood donors in Minna-Nigeria, 2014
Table 2: seroprevalence of anti-CMV IgG and IgM antibodies among blood donors in Minna-Nigeria, 2014
Table 3: socioeconomic-associated factors for CMV (IgG) infection among blood donors in Minna-Nigeria, 2014
Table 4: pattern of Hb and PCV among various strata of blood donors in Minna-Nigeria, 2014
Table 5: variations and statistical significance of means of CMV seropositive and CMV seronegative groups of blood donors in Minna, North Central Nigeria
Figure 1: showing the frequency of CMV seropositivity by age group of the blood donors in Minna, Nigeria
Annex 1: procedure for ELISA technique using ELISA kit (DIALAB® Austria) and Emax ELISA plate reader, E11865 model (Molecular Devices® USA)
- Barret CL, Pretorius JAD. New opportunity for transfusion training for african nurses: development of a distance based blood transfusion short learning programm. African Sang. 2011;14(1):23-8.
- ICRC. Handbook of the International Red Cross and Red Crescent Movement. 2008. Accessed on 15/02/16
- Ahmed SG, Ibrahim UA, Hassan AW. Adequacy and pattern of blood donations in northeast Nigeria: the implications for blood safety. Ann Trop Med Parasitol. 2007;101(8):725-31. PubMed | Google Scholar
- Federal Ministry of Health. The Nigerian National Blood Policy. Nigeria, Abuja. 2006.
- Alao OO, Joseph DE, Mamman A, Banwat EB. The Seroprevalence of cytomegalovirus antibodies among prospective blood donors in Jos. Niger J Med Natl Assoc Resid Dr Niger. 2009;17(2):200-2. Google Scholar
- Chakravarti A, Kashyap B, Matlani M. Cytomegalovirus infection: an Indian perspective. Indian J Med Microbiol. 2007;27(1):3-11. PubMed | Google Scholar
- Tapko JP. The road to a safe blood supply in the African region of the World Health Organization: trends and current status: 1999 - 2006. Afr Sang. 2007;10:1.
- Salawu L, Murainah HA. Pre-donation screening of intending blood donors for antibodies to infectious agents in a Nigerian tertiary health institution: a pilot study. Afr J Med Sci. 2006 Dec;35(4):453-6. PubMed | Google Scholar
- Fowotade A, Agbede O, Salami A, Fayemiwo A, Efunshile A. Cytomegalovirus and HIV co-infection among patients accessing care in a tertiary care centre in Nigeria. Sex Transm Infect. 2013;89:A206-7.
- Ojide CK, Ophori EA, Eghafona NO, Omoti C. Seroprevalence of Cytomegalovirus (CMV) amongst voluntary blood donors in University of Benin Teaching Hospital (UBTH), Edo State, Nigeria. Br J Med Med Res. 2012;2(1):15-20. Google Scholar
- Krajden M et al. Detection of cytomegalovirus in blood donors by PCR using the digene SHARP signal system assay: effects of sample preparation and detection methodology. J Clin Microbiol. 1996;34(1):29-33. Google Scholar
- Oche AO Aminu M. The prevalence of malarial parasitaemia among blood donors in Ahmadu Bello University Teaching Hospital, Shika, Zaria, Nigeria. Niger J Med. 2012;21(4):445-9. PubMed | Google Scholar
- Gargouri J, Elleuch H, Karray H, Rekik H, Hammami A. Prevalence of anti-CMV antibodies in blood donors in the Sfax region (value in blood transfusion). Tunis Med. 2000 Aug-Sep;78(8-9):512-7. PubMed | Google Scholar
- Akinbami AA, Akanmu AS, Adeyemo TA, Wright KO, Dada MO, Dosunmu AO. Cytomegalovirus antibodies among healthy blood donors at Lagos University Teaching Hospital. South African Medical Journal. 2009;99(7):7-9. Google Scholar
- Souza MA, Passos AM, Treitinger A, Spada C. Seroprevalence of cytomegalovirus antibodies in blood donors in southern, Brazil. Rev Soc Bras Med Trop. 2010 Jul-Aug;43(4):359-61. PubMed | Google Scholar
- Hamdan HZ, Abdelbagi IE, Nasser NM, Adam I. Seroprevalence of cytomegalovirus and rubella among pregnant women in western Sudan. Virol J. 2011 May 11;8:217. PubMed | Google Scholar
- Chaudhari CN, Bindra MS. Seroprevalence of Cytomegalovirus among voluntary blood donors. MJAFI. 2009;65(3):252-4. PubMed | Google Scholar
- Wujcicka W, Gaj Z, Wilczynski J, Sobala W, Spiewak E, Nowakowska D. Impact of socioeconomic risk factors on the seroprevalence of cytomegalovirus infections in a cohort of pregnant Polish women between 2010 and 2011 Eur J Clin Microbiol Infect Dis. 2014;10(14):2170-3. Epub 2014 Jun 6. PubMed | Google Scholar
- Abdel Messih AY et al. The degree of safety of family replacement donors versus voluntary non-remunerated donors in an Egyptian population: a comparative study. Blood Transfus. 2014;12(2):159-66. Google Scholar
- Dahourou H, Tapko JB, Kienou K, Nebie K, Sanou M. Recruitment of blood donors in Burkina Faso: how to avoid donations from family members.? Biologicals. 2010 Jan;38(1):39-42. Epub 2010 Feb 9. PubMed | Google Scholar
- Irena Seferi, Pal Xhumari, Genc Burazeri. Prevalence of cytomegalovirus in paid and unpaid blood donor population in Tirana. Int J Heal Sci. 2009;2(4):261. Google Scholar
- Hake JM. Child bearing practices in northern Nigeria. Ibadan Univ Press. 1972. 1,5-11.
- Madauci IH, Isah Y DB. Hausa customs. North Niger Publ Co. 1992. 1,12.
- Welten SPM, Redeker A, Franken KL, Benedict CA, Yagita H, Wensveen FM et al. CD27-CD70 costimulation controls T cell immunity during acute and persistent cytomegalovirus infection. J Virol. 2013 Jun;87(12):6851-65. Epub 2013 Apr 10. PubMed | Google Scholar
- Dowd JB, Haan MN, Blythe L, Moore K, Aiello AE. Socioeconomic gradients in immune response to latent infection. Am J Epidemiol. 2008 Jan 1;167(1):112-20. Epub 2007 Sep 14. PubMed | Google Scholar
- US Centers for Disease Control and Prevention Report (CDC). Health, United States, 2013 with special feature on prescription drugs. 2013. Accessed on 08/06/2016
- de Vries JJ, Korver AM, Verkerk PH, Rusman L, Claas EC, Loeber JG, Kroes AC, Vossen AC. Congenital cytomegalovirus infection in the Netherlands: birth prevalence and risk factors. J Med Virol. 2011;83(10):1777-1782. PubMed | Google Scholar
- Karen Fowler B, Robert Pass F. Risk factors for congenital Cytomegalovirus infection in the offspring of young women: exposure to young children and recent onset of sexual activity. Pediatrics. 2006;118(2):286-92. Google Scholar
- Jeremiah ZA, Umoh RE, Adias TC. Subclinical leukopenia in a cross section of Nigerian blood donors. J Blood Med. 2011;2:79-85. Epub 2011 May 11. PubMed | Google Scholar
- Leena MS, Shafee Mohd. Trend and prevalence of transfusion transmitted infections among blood donors in rural teaching institute, South India. J Pathol Nepal. 2012;2:203-6. Google Scholar
- Xu L-H, Fang J-P, Weng W-J, Huang K, Guo H-X, Liu Y et al. Pure red cell aplasia associated with cytomegalovirus and Epstein-Barr virus infection in seven cases of Chinese children. Hematology. 2013 Jan;18(1):56-9. Epub 2012 Nov 19. PubMed | Google Scholar
- National Population Commission (NPC) FR of N. National Demographic and Health Survey (NDHS). CCF Macro Calvert. 2008. 33.
- Taglietti F, Drapeau CM, Grilli E, Capone A, Noto P, Topino S, Petrosillo N. Hemolytic anemia due to acute cytomegalovirus infection in an immunocompetent adult: a case report and review of the literature. J Med Case Rep. 2010;4:334. PubMed | Google Scholar
- Lopo S, Vinagre E, Palminha P, Paixao MT, Nogueira P, Freitas MG. Seroprevalence to cytomegalovirus in the portuguese population, 2002-2003. Euro Surveill. 2011 Jun 23;16(25). pii: 19896. PubMed | Google Scholar
- Gordon-Smith EC MC. Acquired haemolytic anaemias. In: Hoffbrand AV, Catovski D, Tuddenharm EG (eds). Postgraduate haematology. 5th edition. London, UK. Blackwell Publ ltd. 2005. p151-68.
- Almeida-Porada GD, Ascensăo JL. Cytomegalovirus as a cause of pancytopenia. Leuk Lymphoma. 1996 Apr;21(3-4):217-23. PubMed | Google Scholar
- Marsh JC, Ball SE, Cavenagh J, Darbyshire P, Dokal I, Gordon-Smith EC, Keidan J, Laurie A, Martin A, Mercieca J, Killick SB, Stewart R, Yin JA; British Committee for Standards in Haematology. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol. 2009 Oct;147(1):43-70. Epub 2009 Aug 10. PubMed | Google Scholar










